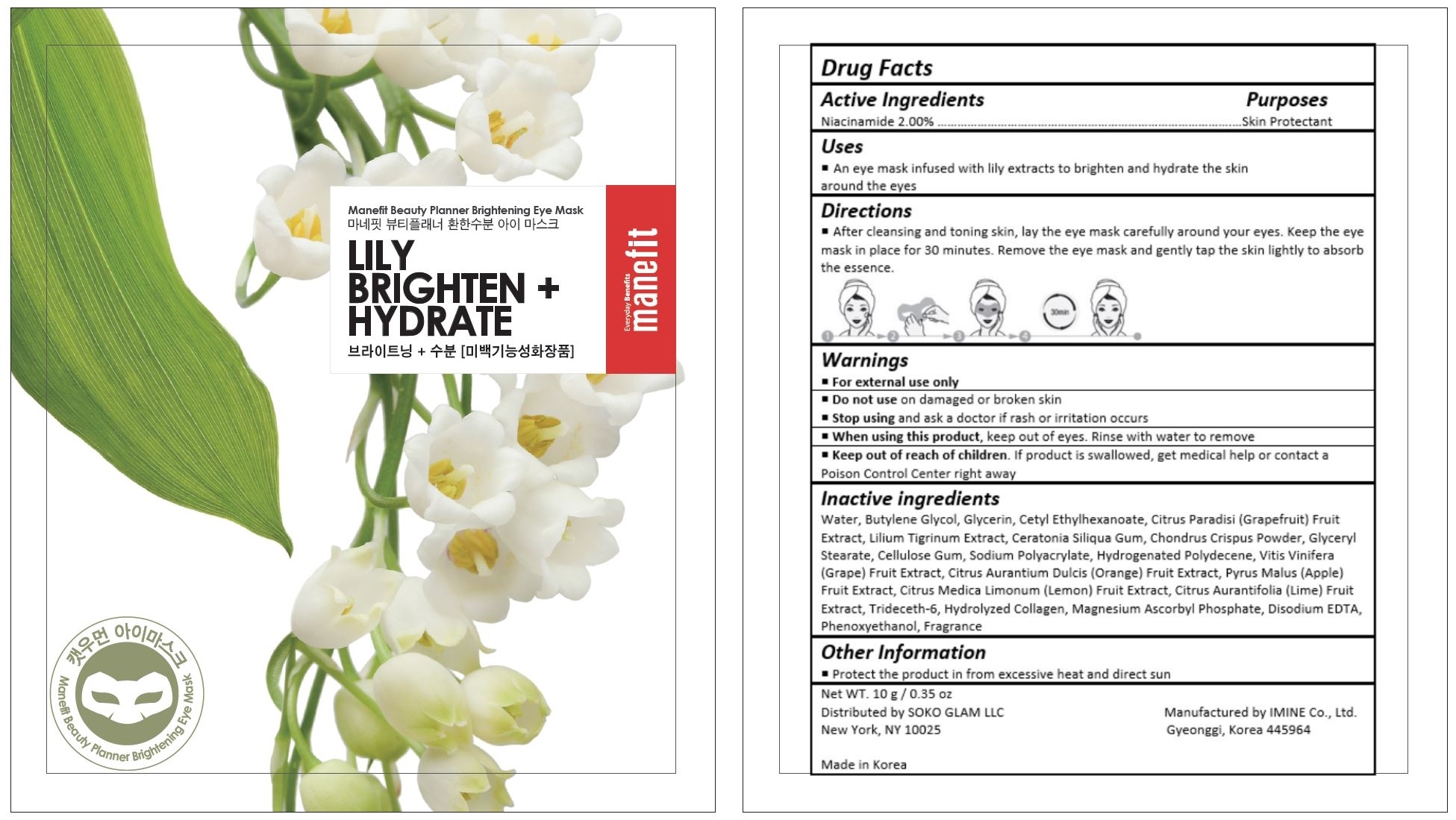
Manefit Beauty Planner Lily Brightening Eye Mask by Imine Co., Ltd. 59401-024_Deactivation
Manefit Beauty Planner Lily Brightening Eye Mask by
Drug Labeling and Warnings
Manefit Beauty Planner Lily Brightening Eye Mask by is a Otc medication manufactured, distributed, or labeled by Imine Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MANEFIT BEAUTY PLANNER LILY BRIGHTENING EYE MASK- niacinamide cream
Imine Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
59401-024_Deactivation
After cleansing and toning skin, lay the eye mask carefully around your eyes. Keep the eye mask in place for 30 minutes. Remove the eye mask and gently tap the skin lightly to absorb the essence.
For external use only.
Do not use on damaged or broken skin.
When using this product, keep out of eyes. Rinse with water to remove
Stop using and ask a doctor if rash occurs.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Water, Butylene Glycol, Glycerin, Cetyl Ethylhexanoate, Citrus Paradisi (Grapefruit) Fruit Extract, Lilium Tigrinum Extract, Ceratonia Siliqua Gum, Chondrus Crispus Powder, Glyceryl Stearate, Cellulose Gum, Sodium Polyacrylate, Hydrogenated Polydecene, Vitis Vinifera (Grape) Fruit Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Pyrus Malus (Apple) Fruit Extract, Citrus Medica Limonum (Lemon) Fruit Extract, Citrus Aurantifolia (Lime) Fruit Extract, Trideceth-6, Hydrolyzed Collagen, Magnesium Ascorbyl Phosphate, Disodium EDTA, Phenoxyethanol, Fragrance
| MANEFIT BEAUTY PLANNER LILY BRIGHTENING EYE MASK
niacinamide cream |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Imine Co., Ltd. (557816813) |
| Registrant - Imine Co., Ltd. (557816813) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| i Mine Co.,Ltd. | 557816813 | manufacture(59401-024) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.