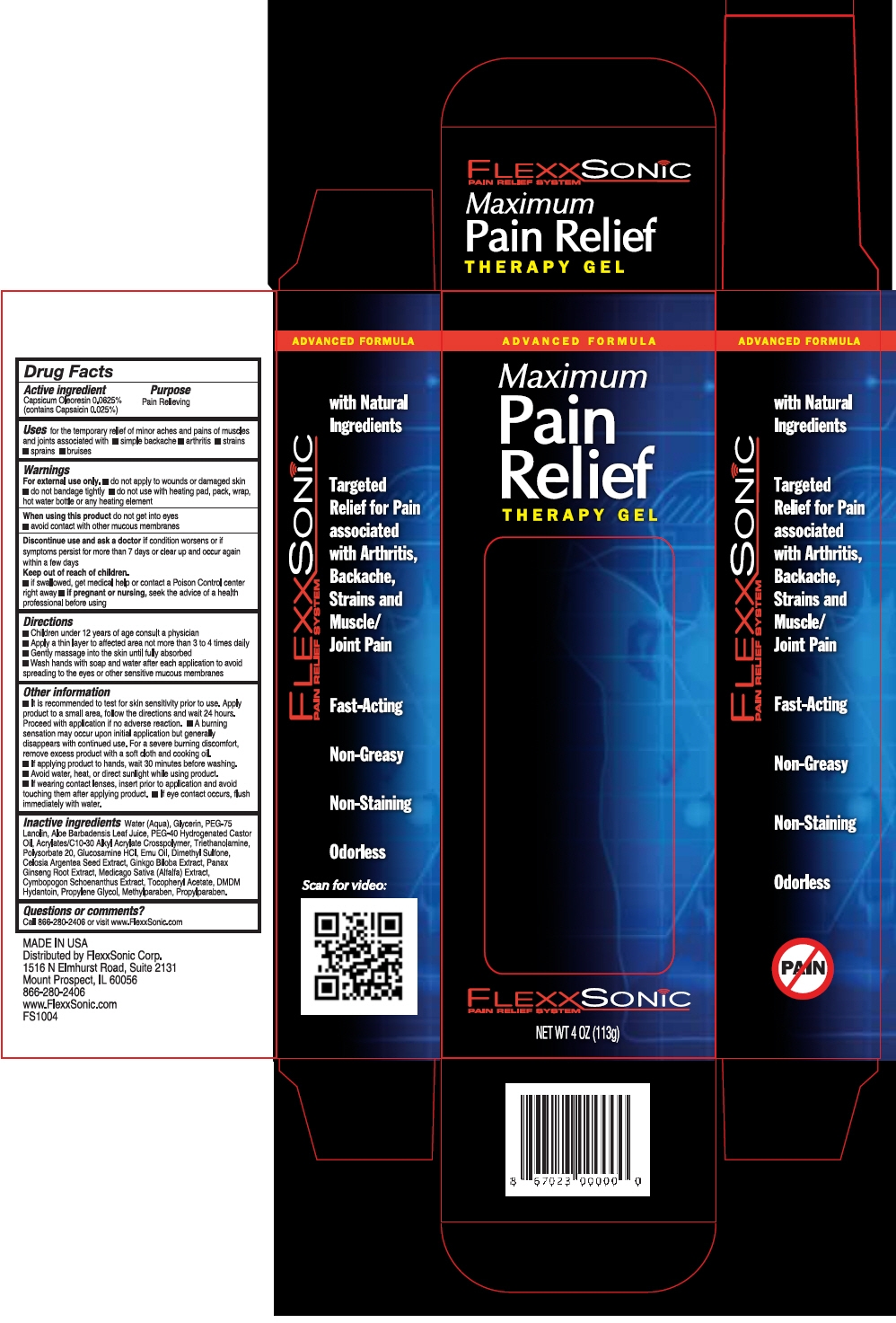
FLEXXSONIC PAIN RELIEF- capsaicin gel
FlexxSonic by
Drug Labeling and Warnings
FlexxSonic by is a Otc medication manufactured, distributed, or labeled by Flexxsonic Corporation, Concept Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
- do not apply to wounds or damaged skin
- do not bandage tightly
- do not use with heating pad, pack, wrap, hot water bottle or any heating element When using this product do not get into eyes
- avoid contact with other mucous membranes Discontinue use and ask a doctor if condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days
- Directions
-
Other information
- It is recommended to test for skin sensitivity prior to use. Apply product to a small area, follow the directions and wait 24 hours. Proceed with application if no adverse reaction.
- A burning sensation may occur upon initial application but generally disappears with continued use. For a severe burning discomfort, remove excess product with a soft cloth and cooking oil.
- If applying product to hands, wait 30 minutes before washing.
- Avoid water, heat, or direct sunlight while using product.
- If wearing contact lenses, insert prior to application and avoid touching them after applying product.
- If eye contact occurs, flush immediately with water.
-
Inactive ingredients
Water (Aqua), Glycerin, PEG-75 Lanolin, Aloe Barbadensis Leaf Juice, PEG-40 Hydrogenated Castor Oil, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Polysorbate 20, Glucosamine HCI, Emu Oil, Dimethyl Sulfone, Celosia Argentea Seed Extract, Ginkgo Biloba Extract, Panax Ginseng Root Extract, Medicago Saliva (Alfalfa) Extract, Cymbopogon Schoenanthus Extract, Tocopheryl Acetate, DMDM Hydantoin, Propylene Glycol, Methylparaben, Propylparaben.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 113 g Bottle Carton
-
INGREDIENTS AND APPEARANCE
FLEXXSONIC PAIN RELIEF
capsaicin gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69397-156 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Capsaicin (UNII: S07O44R1ZM) (Capsaicin - UNII:S07O44R1ZM) Capsaicin 0.25 mg in 1 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) PEG-75 LANOLIN (UNII: 09179OX7TB) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) ALOE VERA LEAF (UNII: ZY81Z83H0X) ALFALFA LEAF (UNII: HY3L927V6M) CYMBOPOGON SCHOENANTHUS LEAF (UNII: XF54B1Z2HF) GLUCOSAMINE HYDROCHLORIDE (UNII: 750W5330FY) GINKGO (UNII: 19FUJ2C58T) POLYSORBATE 20 (UNII: 7T1F30V5YH) EMU OIL (UNII: 344821WD61) DMDM HYDANTOIN (UNII: BYR0546TOW) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) ASIAN GINSENG (UNII: CUQ3A77YXI) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69397-156-44 113 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 07/01/2015 Labeler - Flexxsonic Corporation (079378932) Establishment Name Address ID/FEI Business Operations Concept Laboratories, Inc. 962282612 MANUFACTURE(69397-156)
Trademark Results [FlexxSonic]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FLEXXSONIC 86824921 not registered Dead/Abandoned |
Flexxsonic Corporation 2015-11-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.