These highlights do not include all the information needed to use TIZANIDINE TABLETS safely and effectively. See full prescribing information for TIZANIDINE TABLETS.TIZANIDINE tablets, for oral useInitial U.S. Approval: 1996
tizanidine by
Drug Labeling and Warnings
tizanidine by is a Prescription medication manufactured, distributed, or labeled by Graviti Pharmaceuticals Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TIZANIDINE- tizanidine tablet
Graviti Pharmaceuticals Private Limited
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TIZANIDINE TABLETS safely and effectively. See full prescribing information for TIZANIDINE TABLETS.
TIZANIDINE tablets, for oral use Initial U.S. Approval: 1996 INDICATIONS AND USAGETizanidine tablet is a central alpha-2-adrenergic agonist indicated for the management of spasticity. Because of the short duration of therapeutic effect, treatment with tizanidine tablets should be reserved for those daily activities and times when relief of spasticity is most important. (1) DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONSWARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse reactions (greater than 2% of 264 patients taking tizanidine and greater than in placebo-treated patients in three multiple dose, placebo-controlled studies) were dry mouth, somnolence, asthenia, dizziness, urinary tract infection, constipation, liver function tests abnormal, vomiting, speech disorder, amblyopia, urinary frequency, flu syndrome, SGPT/ALT increased, dyskinesia, nervousness, pharyngitis, and rhinitis (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Graviti Pharmaceuticals Inc., at 1-855-298-4506 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. USE IN SPECIFIC POPULATIONSSee 17 for PATIENT COUNSELING INFORMATION. Revised: 8/2024 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Tizanidine tablets is indicated for the management of spasticity. Because of the short duration of therapeutic effect, treatment with tizanidine tablets should be reserved for those daily activities and times when relief of spasticity is most important [see Dosage and Administration (2.1)].
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
Tizanidine tablets may be prescribed with or without food. Once the formulation has been selected and the decision to take with or without food has been made, this regimen should not be altered.
Food has complex effects on tizanidine pharmacokinetics, which differ with the different formulations. Zanaflex®Capsules and tizanidine tablets are bioequivalent to each other under fasting conditions (more than 3 hours after a meal), but not under fed conditions (within 30 minutes of a meal). These pharmacokinetic differences may result in clinically significant differences when switching administration of tablet and capsules and when switching administration between the fed or fasted state. These changes may result in increased adverse events, or delayed or more rapid onset of activity, depending upon the nature of the switch. For this reason, the prescriber should be thoroughly familiar with the changes in kinetics associated with these different conditions [see Clinical Pharmacology (12.3)].
The recommended starting dose is 2 mg. Because the effect of tizanidine tablets peaks at approximately 1 to 2 hours post-dose and dissipates between 3 to 6 hours post-dose, treatment can be repeated at 6 to 8 hour intervals, as needed, to a maximum of three doses in 24 hours.
Dosage can be gradually increased by 2 mg to 4 mg at each dose, with 1 to 4 days between dosage increases, until a satisfactory reduction of muscle tone is achieved. The total daily dose should not exceed 36 mg. Single doses greater than 16 mg have not been studied.
2.2 Dosing in Patients with Renal Impairment
Tizanidine tablets should be used with caution in patients with renal insufficiency (creatinine clearance < 25 mL/min), as clearance is reduced by more than 50%. In these patients, during titration, the individual doses should be reduced. If higher doses are required, individual doses rather than dosing frequency should be increased [see Warnings and Precautions (5.7)].
2.3 Dosing in Patients with Hepatic Impairment
Tizanidine tablets should be used with caution in patients with any hepatic impairment. In these patients, during titration, the individual doses should be reduced. If higher doses are required, individual doses rather than dosing frequency should be increased. Monitoring of aminotransferase levels is recommended for baseline and 1 month after maximum dose is achieved, or if hepatic injury is suspected. [see Use in Specific Populations (8.7)]
2.4 Drug Discontinuation
If therapy needs to be discontinued, particularly in patients who have been receiving high doses (20 mg to 36 mg daily) for long periods (9 weeks or more) or who may be on concomitant treatment with narcotics, the dose should be decreased slowly (2 mg to 4 mg per day) to minimize the risk of withdrawal and rebound hypertension, tachycardia, and hypertonia [see Drug Abuse and Dependence (9.3)].
3 DOSAGE FORMS AND STRENGTHS
2 mg - white to off-white, round uncoated tablets debossed with "39" on the other side and functionally scored on the other side.
4 mg- white to off-white, round uncoated tablets with functionally scored one side and debossed with "40" on the other side.
4 CONTRAINDICATIONS
Tizanidine tablets is contraindicated in patientstaking potent inhibitors of CYP1A2, such as fluvoxamine or ciprofloxacin [see Drug Interactions (7.1, 7.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
Tizanidine is an α2-adrenergic agonist that can produce hypotension. Syncope has been reported in the post marketing setting. The chance of significant hypotension may possibly be minimized by titration of the dose and by focusing attention on signs and symptoms of hypotension prior to dose advancement. In addition, patients moving from a supine to fixed upright position may be at increased risk for hypotension and orthostatic effects.
Monitor for hypotension when tizanidine tablets is used in patients receiving concurrent antihypertensive therapy. It is not recommended that tizanidine tablets be used with other α2- adrenergic agonists. Clinically significant hypotension (decreases in both systolic and diastolic pressure) has been reported with concomitant administration of either fluvoxamine or ciprofloxacin and single doses of 4 mg of tizanidine tablets. Therefore, concomitant use of tizanidine tablets with fluvoxamine or with ciprofloxacin, potent inhibitors of CYP1A2, is contraindicated [see Contraindications (4) and Drug Interactions (7.1, 7.2)].
5.2 Risk of Liver Injury
Tizanidine tablets may cause hepatocellular liver injury. Tizanidine tablets should be used with caution in patients with any hepatic impairment. Monitoring of aminotransferase levels is recommended for baseline and 1 month after maximum dose is achieved, or if hepatic injury is suspected. [see Dosage and Administration (2.3) and Use in Specific Populations (8.7)]
5.3 Sedation
Tizanidine tablets can cause sedation, which may interfere with everyday activity. In the multiple dose studies, the prevalence of patients with sedation peaked following the first week of titration and then remained stable for the duration of the maintenance phase of the study. The CNS depressant effects of tizanidine tablets with alcohol and other CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants) may be additive. Monitor patients who take tizanidine tablets with another CNS depressant for symptoms of excess sedation. [see Drug Interactions (7.5, 7.6)]
5.4 Hallucinosis/Psychotic-Like Symptoms
Tizanidine tablets use has been associated with hallucinations. Formed, visual hallucinations or delusions have been reported in 5 of 170 patients (3%) in two North American controlled clinical studies. Most of the patients were aware that the events were unreal. One patient developed psychosis in association with the hallucinations. One patient among these 5 continued to have problems for at least 2 weeks following discontinuation of tizanidine. Consider discontinuing tizanidine tablets in patients who develop hallucinations.
5.5 Interaction with CYP1A2 Inhibitors
Because of potential drug interactions, Tizanidine tablets is contraindicated in patients taking potent CYP1A2 inhibitors, such as fluvoxamine or ciprofloxacin. Adverse reactions such as hypotension, bradycardia, or excessive drowsiness can occur when tizanidine tablets is taken with other CYP1A2 inhibitors, such as zileuton, fluoroquinolones other than ciprofloxacin (which is contraindicated), antiarrhythmics (amiodarone, mexiletine, propafenone), cimetidine, famotidine, oral contraceptives, acyclovir, and ticlopidine). Concomitant use should be avoided unless the necessity for tizanidine tablets therapy is clinically evident. In such a case, use with caution. [see Drug Interactions (7.3) and Clinical Pharmacology (12.3)]
5.6 Hypersensitivity Reactions
Tizanidine tablets can cause anaphylaxis. Signs and symptoms including respiratory compromise, urticaria, and angioedema of the throat and tongue have been reported. Patients should be informed of the signs and symptoms of severe allergic reactions and instructed to discontinue tizanidine tablets and seek immediate medical care should these signs and symptoms occur. [see Contraindications (4)]
5.7 Increased Risk of Adverse Reactions in Patients with Renal Impairment
Tizanidine tablets should be used with caution in patients with renal insufficiency (creatinine clearance < 25 mL/min), as clearance is reduced by more than 50%. In these patients, during titration, the individual doses should be reduced. If higher doses are required, individual doses rather than dosing frequency should be increased. These patients should be monitored closely for the onset or increase in severity of the common adverse events (dry mouth, somnolence, asthenia and dizziness) as indicators of potential overdose. [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)]
5.8 Withdrawal Adverse Reactions
Withdrawal adverse reactions include rebound hypertension, tachycardia, and hypertonia. To minimize the risk of these reactions, particularly in patients who have been receiving high doses (20 to 28 mg daily) for long periods of time (9 weeks or more) or who may be on concomitant treatment with narcotics, the dose should be decreased slowly (2 to 4 mg per day). [see Dosage and Administration (2.2)]
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in other sections of the prescribing information:
- Hypotension[see Warnings and Precautions (5.1)]
- Liver Injury [see Warnings and Precautions (5.2)]
- Sedation [see Warnings and Precautions (5.3)]
- Hallucinosis/Psychotic-Like Symptoms [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Three double-blind, randomized, placebo controlled -clinical studies were conducted to evaluate the effect of tizanidine on spasticity control. Two studies were conducted in patients with multiple sclerosis and one in patients with spinal cord injury. Each study had a 13-week active treatment period which included a 3-week titration phase to the maximum tolerated dose up to 36 mg/day in three divided doses, a 9-week plateau phase where the dose of tizanidine was held constant and a 1- week dose tapering. In all, 264 patients received tizanidine and 261 patients received placebo. Across the three studies patient ages ranged from 15 to 69 years and 51.4 percent were women. The median dose during the plateau phase ranged from 20 to 28 mg/day.
The most frequent adverse reactions reported in multiple dose, placebo-controlled clinical studies involving 264 patients with spasticity were dry mouth, somnolence/sedation, asthenia (weakness, fatigue and/or tiredness) and dizziness. Three-quarters of the patients rated the events as mild to moderate and one-quarter of the patients rated the events as being severe. These events appeared to be dose related.
Table 1 lists signs and symptoms that were reported in greater than 2% of patients in three multiple dose, placebo-controlled studies who received tizanidine tablets where the frequency in the tizanidine tablets group was greater than the placebo group. For comparison purposes, the corresponding frequency of the event (per 100 patients) among placebo treated patients is also provided.
Table 1: Multiple Dose, Placebo-Controlled Studies—Frequent (>2%) Adverse Reactions Reported for Which Tizanidine Tablets Incidence is Greater than Placebo
|
Event | Placebo
N = 261 % | Tizanidine Tablet N = 264
% |
| Dry mouth | 10 | 49 |
| Somnolence | 10 | 48 |
| Asthenia* | 16 | 41 |
| Dizziness | 4 | 16 |
| UTI | 7 | 10 |
| Infection | 5 | 6 |
| Constipation | 1 | 4 |
| Liver test abnormality | 2 | 6 |
| Vomiting | 0 | 3 |
| Speech disorder | 0 | 3 |
| Amblyopia (blurred vision) | <1 | 3 |
| Urinary frequency | 2 | 3 |
| Flu syndrome | 2 | 3 |
| Dyskinesia | 0 | 3 |
| Nervousness | <1 | 3 |
| Pharyngitis | 1 | 3 |
| Rhinitis | 2 | 3 |
*(weakness, fatigue, and/or tiredness)
In the single dose, placebo-controlled study involving 142 patients with spasticity due to multiple sclerosis (Study 1) [see Clinical Studies (14)], the patients were specifically asked if they had experienced any of the four most common adverse reactions: dry mouth, somnolence (drowsiness), asthenia (weakness, fatigue and/or tiredness) and dizziness. In addition, hypotension and bradycardia were observed. The occurrence of these reactions is summarized in Table 2. Other events were, in general, reported at a rate of 2% or less.
Table 2: Single Dose, Placebo-Controlled Study—Common Adverse Reactions Reported
|
Event | Placebo
N = 48 % | Tizanidine Tablet, 8mg, N = 45
% | Tizanidine Tablets,
16 mg, N = 49 % |
| Somnolence | 31 | 78 | 92 |
| Dry mouth | 35 | 76 | 88 |
| Asthenia* | 40 | 67 | 78 |
| Dizziness | 4 | 22 | 45 |
| Hypotension | 0 | 16 | 33 |
| Bradycardia | 0 | 2 | 10 |
6.2 Post-Marketing Experience
The following adverse reactions have been identified during post approval use of tizanidine tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Certain events, such as somnolence, dry mouth, hypotension, decreased blood pressure, bradycardia, dizziness, weakness or asthenia, muscle spasms, hallucinations, fatigue, liver function test abnormality and hepatotoxicity, have been observed in post marketing and clinical trials and are discussed in previous sections of this document.
The following adverse reactions have been identified as occurring in the post marketing experience of tizanidine tablets. Based on the information provided regarding these reactions, a causal relationship with tizanidine tablets cannot be entirely excluded. The events are listed in order of decreasing clinical significance; severity in the post marketing setting is not reported.
- Stevens Johnson Syndrome
- Anaphylactic Reaction
- Exfoliative Dermatitis
- Ventricular Tachycardia
- Hepatitis
- Convulsion
- Depression
- Arthralgia
- Paresthesia
- Rash
- Tremor
7 DRUG INTERACTIONS
7.1 Fluvoxamine
Concomitant use of fluvoxamine and tizanidine tablets is contraindicated. Changes in pharmacokinetics of tizanidine when administered with fluvoxamine resulted in significantly decreased blood pressure, increased drowsiness, and increased psychomotor impairment. [see Contraindications (4) and Clinical Pharmacology (12.3)]
7.2 Ciprofloxacin
Concomitant use of ciprofoxacin and tizanidine tablets is contraindicated. Changes in pharmacokinetics of tizanidine when administered with ciprofloxacin resulted in significantly decreased blood pressure, increased drowsiness, and increased psychomotor impairment [See Contraindications (4) and Clinical Pharmacology (12.3)]
7.3 CYP1A2 Inhibitors other than Fluvoxamine and Ciprofloxacin
Because of potential drug interactions, concomitant use of tizanidine tablets with other CYP1A2 inhibitors, such as zileuton, fluoroquinolones other than strong CYP1A2 inhibitors (which are contraindicated), antiarrhythmics (amiodarone, mexiletine, propafenone, and verapamil), cimetidine, famotidine, oral contraceptives, acyclovir, and ticlopidine) should be avoided. If their use is clinically necessary, therapy should be initiated with 2 mg dose and increased in 2–4 mg steps daily based on patient response to therapy. If adverse reactions such as hypotension, bradycardia, or excessive drowsiness occur, reduce or discontinue tizanidine tablets therapy. [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)]
7.4 Oral Contraceptives
Concomitant use of tizanidine tablets with oral contraceptives is not recommended. However, if concomitant use is clinically necessary, initiate tizanidine tablets with a single 2 mg dose and increase in 2 to 4 mg steps daily based on patient response to therapy. If adverse reactions such as hypotension, bradycardia, or excessive drowsiness occur, reduce or discontinue tizanidine tablets therapy. [see Clinical Pharmacology (12.3)]
7.5 Alcohol
Alcohol increases the overall amount of drug in the bloodstream after a dose of tizanidine tablets. This was associated with an increase in adverse reactions of tizanidine tablets. The CNS depressant effects of tizanidine tablets and alcohol are additive. [see Clinical Pharmacology (12.3)]
7.6 Other CNS Depressants
The sedative effects of tizanidine tablets with CNS depressants (e.g., benzodiazepines, opioids, tricyclic antidepressants) may be additive. Monitor patients who take tizanidine tablets with another CNS depressant for symptoms of excess sedation. [see Clinical Pharmacology (12.3)]
7.7 a2-Adrenergic Agonists
Because hypotensive effects may be cumulative, it is not recommended that tizanidine tablets be used with other α2-adrenergic agonists. [see Warnings and Precautions (5.1)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
There are no adequate data on the developmental risk associated with use of tizanidine tablets in pregnant women. In animal studies, administration of tizanidine during pregnancy resulted in developmental toxicity (embryofetal and postnatal offspring mortality and growth deficits) at doses less than those used clinically, which were not associated with maternal toxicity (see Animal Data).
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% - 4% and 15% - 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
Oral administration of tizanidine (0.3 to 100 mg/kg/day) to pregnant rats during the period of organogenesis resulted in embryofetal and postnatal offspring mortality and reductions in body weight at doses of 30 mg/kg/day and above. Maternal toxicity was observed at the highest dose tested. The no-effect dose for embryofetal developmental toxicity in rats (3 mg/kg/day) is similar to the maximum recommended human dose (MRHD) of 36 mg/day on a body surface area (mg/m2) basis.
Oral administration of tizanidine (1 to 100 mg/kg/day) to pregnant rabbits during the period of organogenesis resulted in embryofetal and postnatal offspring mortality at all doses. Maternal toxicity was observed at the highest dose tested. Oral administration of tizanidine (10 and 30 mg/kg/day) during the perinatal period of pregnancy (2-6 days prior to delivery) resulted in increased postnatal offspring mortality at both doses. A no effect dose for embryofetal developmental toxicity in rabbit was not identified. The lowest dose tested (1 mg/kg/day) is less than the MRHD on a mg/m basis
In a pre- and postnatal development study in rats, oral administration of tizanidine (3 to 30 mg/kg/day) resulted in increased postnatal offspring mortality. A no-effect dose for pre- and postnatal developmental toxicity was not identified. The lowest dose tested (3 mg/kg/day) is similar to the MRHD on a mg/m2 basis, respectively.
8.2 Lactation
There are no data on the presence of tizanidine in human milk, the effects on the breastfed infant, or the effects on human milk production. Animal studies have reported the presence of tizanidine in the milk of lactating animals.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for tizanidine tablets and any potential adverse effects on the breastfed infant from tizanidine tablets or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
There are no adequate and well-controlled studies in humans on the effect of tizanidine tablets on female or male reproductive potential. Oral administration of tizanidine to male and female rats resulted in adverse effects on fertility [see Nonclinical Toxicology (13.1)].
8.5 Geriatric Use
Tizanidine tablets is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Clinical studies of tizanidine tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently than younger subjects. Cross- study comparison of pharmacokinetic data following single dose administration of 6 mg tizanidine tablets showed that younger subjects cleared the drug four times faster than the elderly subjects. In elderly patients with renal insufficiency (creatinine clearance <25 mL/min), tizanidine clearance is reduced by more than 50% compared to healthy elderly subjects; this would be expected to lead to a longer duration of clinical effect. During titration, the individual doses should be reduced. If higher doses are required, individual doses rather than dosing frequency should be increased. Monitor elderly patients because they may have an increased risk for adverse reactions associated with tizanidine tablets.
8.6 Impaired Renal Function
Tizanidine tablets is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. In patients with renal insufficiency (creatinine clearance < 25 mL/min) clearance was reduced by more than 50%. In these patients, individual doses should be reduced during titration. If higher doses are required, individual doses rather than dosing frequency should be increased. These patients should be monitored closely for the onset or increase in severity of tizanidine common adverse events (dry mouth, somnolence, asthenia and dizziness) as indicators of potential overdosage. [see Dosage and Administration (2.2), Warnings and Precautions (5.7) and Clinical Pharmacology (12.3)]
8.7 Impaired Hepatic Function
The influence of hepatic impairment on the pharmacokinetics of tizanidine has not been evaluated. Because tizanidine is extensively metabolized in the liver, hepatic impairment would be expected to have significant effects on pharmacokinetics of tizanidine. [see Dosing and Administration (2.3), Warnings and Precautions (5.2), and Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Abuse potential was not evaluated in human studies. Rats were able to distinguish tizanidine from saline in a standard discrimination paradigm, after training, but failed to generalize the effects of morphine, cocaine, diazepam, or phenobarbital to tizanidine.
9.3 Dependence
Tizanidine is closely related to clonidine, which is often abused in combination with narcotics and is known to cause symptoms of rebound upon abrupt withdrawal. Three cases of rebound symptoms on sudden withdrawal of tizanidine have been reported. The case reports suggest that these patients were also misusing narcotics. Withdrawal symptoms included hypertension, tachycardia, hypertonia, tremor, and anxiety. Withdrawal symptoms are more likely to occur in cases where high doses are used, especially for prolonged periods, or with concomitant use of narcotics. If therapy needs to be discontinued, the dose should be decreased slowly to minimize the risk of withdrawal symptoms [see Dosage and Administration (2.2)].
Monkeys were shown to self-administer tizanidine in a dose-dependent manner, and abrupt cessation of tizanidine produced transient signs of withdrawal at doses > 35 times the maximum recommended human dose on a mg/m2 basis. These transient withdrawal signs (increased locomotion, body twitching, and aversive behavior toward the observer) were not reversed by naloxone administration.
10 OVERDOSAGE
A review of the safety surveillance database revealed cases of intentional and accidental tizanidine tablets overdose. Some of the cases resulted in fatality and many of the intentional overdoses were with multiple drugs including CNS depressants. The clinical manifestations of tizanidine overdose were consistent with its known pharmacology. In the majority of cases a decrease in sensorium was observed including lethargy, somnolence, confusion and coma. Depressed cardiac function is also observed including most often bradycardia and hypotension. Respiratory depression is another common feature of tizanidine overdose.
Should overdose occur, basic steps to ensure the adequacy of an airway and the monitoring of cardiovascular and respiratory systems should be undertaken. Tizanidine is a lipid-soluble drug, which is only slightly soluble in water and methanol. Therefore, dialysis is not likely to be an efficient method of removing drug from the body. In general, symptoms resolve within one to three days following discontinuation of tizanidine and administration of appropriate therapy. Due to the similar mechanism of action, symptoms and management of tizanidine overdose are similar to that following clonidine overdose. For the most recent information concerning the management of overdose, contact a poison control center.
11 DESCRIPTION
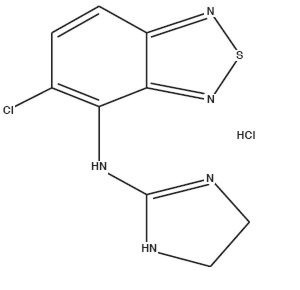
Tizanidine Hydrochloride, USP is a central alpha2-adrenergic agonist. Tizanidine Hydrochloride USP is a white to slightly yellow crystalline powder. Tizanidine Hydrochloride USP is sparingly soluble in water and methanol. Its chemical name is 5- chloro-4-(2- imidazolin-2-ylamino)-2,1,3-benzothiadiazole monohydrochloride. Tizanidine's molecular formula is C9H8ClN5S-HCl, its molecular weight is 290.2 and its structural formula is:
Tizanidine tablets are supplied as 2 mg and 4 mg tablets for oral administration. Tizanidine tablets contain the active ingredient, tizanidine hydrochloride USP (2.288 mg of tizanidine hydrochloride is equivalent to 2 mg tizanidine base, 4.576 mg of tizanidine hydrochloride is equivalent to 4 mg tizanidine base), and the inactive ingredients, colloidal silicon dioxide, stearic acid, microcrystalline cellulose and anhydrous lactose.
The drug product meets the requirements of USP Dissolution Test 2.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of action
Tizanidine is a central alpha-2-adrenergic receptor agonist and presumably reduces spasticity by increasing presynaptic inhibition of motor neurons. The effects of tizanidine are greatest on polysynaptic pathways. The overall effect of these actions is thought to reduce facilitation of spinal motor neurons.
12.3 Pharmacokinetics
Following oral administration, tizanidine is essentially completely absorbed. The absolute oral bioavailability of tizanidine is approximately 40% (CV = 24%), due to extensive first-pass hepatic metabolism. Tizanidine is extensively distributed throughout the body with a mean steady state volume of distribution of 2.4 L/kg (CV = 21%) following intravenous administration in healthy adult volunteers. Tizanidine is approximately 30% bound to plasma proteins.
Differences between Zanaflex® Capsules and tizanidine tablets
Zanaflex® Capsules and tizanidine tablets are bioequivalent to each other under fasting conditions, but not under fed conditions. A single dose of either two 4 mg tablets or two 4 mg capsules was administered under fed and fasting conditions in an open label, four period, randomized crossover study in 96 human volunteers, of whom 81 were eligible for the statistical analysis. Following oral administration of either the tablet or capsule (in the fasted state), peak plasma concentrations of tizanidine occurred 1.0 hours after dosing with a half-life of approximately 2 hours. When two 4 mg tablets were administered with food, the mean maximal plasma concentration was increased by approximately 30%, and the median time to peak plasma concentration was increased by 25 minutes, to 1 hour and 25 minutes. In contrast, when two 4 mg capsules were administered with food, the mean maximal plasma concentration was decreased by 20%, the median time to peak plasma concentration was increased 2 to 3 hours. Consequently, the mean Cmax for the capsule when administered with food is approximately 66% the Cmax for the tablet when administered with food.
Food also increased the extent of absorption for both the tablets and capsules. The increase with the tablet (~30%) was significantly greater than with the capsule (~10%). Consequently when each was administered with food, the amount absorbed from the capsule was about 80% of the amount absorbed from the tablet. Administration of the capsule contents sprinkled on applesauce was not bioequivalent to administration of an intact capsule under fasting conditions. Administration of the capsule contents on applesauce resulted in a 15%–20% increase in Cmax and AUC of tizanidine and a 15 minute decrease in the median lag time and time to peak concentration compared to administration of an intact capsule while fasting.
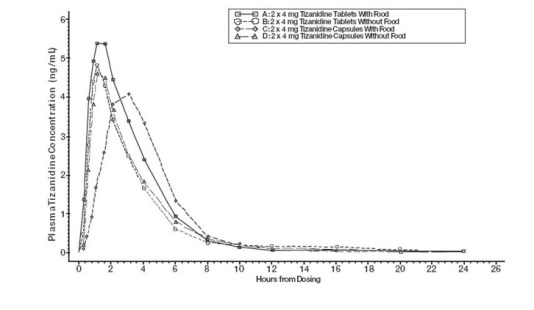
Figure 1: Mean Tizanidine Concentration vs. Time Profiles For Tizanidine Tablets and Capsules (2 × 4 mg) Under Fasted and Fed Conditions
Metabolism and Excretion
Tizanidine has linear pharmacokinetics over the doses studied in clinical development (1–20 mg). Tizanidine has a half-life of approximately 2.5 hours (CV=33%). Approximately 95% of an administered dose is metabolized. The primary cytochrome P450 isoenzyme involved in tizanidine metabolism is CYP1A2. Tizanidine metabolites are not known to be active; their half-lives range from 20 to 40 hours.
Following single and multiple oral dosing of 14C-tizanidine, an average of 60% and 20% of total radioactivity was recovered in the urine and feces, respectively.
Specific Populations
Age Effects
No specific pharmacokinetic study was conducted to investigate age effects. Cross study comparison of pharmacokinetic data following single dose administration of 6 mg tizanidine tablets showed that younger subjects cleared the drug four times faster than the elderly subjects. Tizanidine tablets has not been evaluated in children. [see Use in Specific Populations (8.4, 8.5)]
Hepatic Impairment
The influence of hepatic impairment on the pharmacokinetics of tizanidine has not been evaluated. Because tizanidine is extensively metabolized in the liver, hepatic impairment would be expected to have significant effects on pharmacokinetics of tizanidine. Tizanidine tablets is not recommended in this patient population [see Use in Specific Populations (8.7)]
Renal Impairment
Tizanidine clearance is reduced by more than 50% in elderly patients with renal insufficiency (creatinine clearance < 25 mL/min) compared to healthy elderly subjects; this would be expected to lead to a longer duration of clinical effect. Tizanidine tablets should be used with caution in renally impaired patients [see Warnings and Precautions (5.7) and Use in Specific Populations (8.6)].
Gender Effects
No specific pharmacokinetic study was conducted to investigate gender effects. Retrospective analysis of pharmacokinetic data, however, following single and multiple dose administration of 4 mg tizanidine tablets showed that gender had no effect on the pharmacokinetics of tizanidine.
Race Effects
Pharmacokinetic differences due to race have not been studied.
Drug Interactions
CYP1A2 Inhibitors
The interaction between tizanidine tablets and either fluvoxamine or ciprofloxacin is most likely due to inhibition of CYP1A2 by fluvoxamine or ciprofloxacin. The effect of fluvoxamine on the pharmacokinetics of a single 4 mg dose of tizanidine tablets was studied in 10 healthy subjects. The Cmax, AUC, and half-life of tizanidine increased by 12- fold, 33-fold, and 3-fold, respectively. The effect of ciprofloxacin on the pharmacokinetics of a single 4 mg dose of tizanidine tablets was studied in 10 healthy subjects. The Cmax and AUC of tizanidine increased by 7-fold and 10-fold, respectively. [see Contraindications (4)]
Although there have been no clinical studies evaluating the effects of other CYP1A2 inhibitors on tizanidine, other CYP1A2 inhibitors, such as zileuton, other fluoroquinolones, antiarrhythmics (amiodarone, mexiletine, propafenone and verapamil), cimetidine, famotidine oral contraceptives, acyclovir and ticlopidine, may also lead to substantial increases in tizanidine blood concentrations [see Warnings and Precautions (5.5)].
In vitro studies of cytochrome P450 isoenzymes using human liver microsomes indicate that neither tizanidine nor the major metabolites are likely to affect the metabolism of other drugs metabolized by cytochrome P450 isoenzymes.
Oral Contraceptives
No specific pharmacokinetic study was conducted to investigate interaction between oral contraceptives and tizanidine tablets. Retrospective analysis of population pharmacokinetic data following single and multiple dose administration of 4 mg tizanidine tablets, however, showed that women concurrently taking oral contraceptives had 50% lower clearance of tizanidine compared to women not on oral contraceptives [see Warnings and Precautions (5.5)].
Acetaminophen
Tizanidine delayed the Tmax of acetaminophen by 16 minutes. Acetaminophen did not affect the pharmacokinetics of tizanidine.
Alcohol
Alcohol increased the AUC of tizanidine by approximately 20%, while also increasing its Cmax by approximately 15%. This was associated with an increase in side effects of tizanidine. The CNS depressant effects of tizanidine and alcohol are additive.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, mutagenesis, impairment of fertility
Tizanidine was administered to mice for 78 weeks at oral doses up to 16 mg/kg/day, which is 2 times the maximum recommended human dose (MRHD) of 36 mg/day on a body surface area (mg/m ) basis. Tizanidine was administered to rats for 104 weeks at oral doses up to 9 mg/kg/day, which is 2.5 times the MRHD on a mg/m2 basis. There was no increase in tumors in either species.
Mutagenesis
Tizanidine was negative in in vitro (bacterial reverse mutation [Ames] , mammalian gene mutation, and chromosomal aberration test in mammalian cells) and in vivo (bone marrow micronucleus, and cytogenetics) assay.
Impairment of fertility
Oral administration of tizanidine to rats prior to and during mating and continuing during early pregnancy in females resulted in reduced fertility in male and female rats at doses of 30 and 10 mg/kg/day, respectively. No effect on fertility was observed at doses of 10 (male) and 3 (female) mg/kg/day, which are approximately 3 times and similar to the MRHD, respectively, on a mg/m basis.
14 CLINICAL STUDIES
Tizanidine's capacity to reduce increased muscle tone associated with spasticity was demonstrated in two adequate and well controlled studies in patients with multiple sclerosis or spinal cord injury (Studies 1 and 2).
Single-Dose Study in Patients with Multiple Sclerosis with Spasticity
In Study 1, patients with multiple sclerosis were randomized to receive single oral doses of drug or placebo. Patients and assessors were blind to treatment assignment and efforts were made to reduce the likelihood that assessors would become aware indirectly of treatment assignment (e.g., they did not provide direct care to patients and were prohibited from asking questions about side effects). In all, 140 patients received placebo, 8 mg or 16 mg of tizanidine tablets.
Response was assessed by physical examination; muscle tone was rated on a 5 point scale (Ashworth score), with a score of 0 used to describe normal muscle tone. A score of 1 indicated a slight spastic catch while a score of 2 indicated more marked muscle resistance. A score of 3 was used to describe considerable increase in tone, making passive movement difficult. A muscle immobilized by spasticity was given a score of 4. Spasm counts were also collected.
Assessments were made at 1, 2, 3 and 6 hours after treatment. A statistically significant reduction of the Ashworth score for tizanidine tablets compared to placebo was detected at 1, 2 and 3 hours after treatment. Figure 2 below shows a comparison of the mean change in muscle tone from baseline as measured by the Ashworth scale. The greatest reduction in muscle tone was 1 to 2 hours after treatment. By 6 hours after treatment, muscle tone in the 8 and 16 mg tizanidine tablets groups was indistinguishable from muscle tone in placebo treated patients. Within a given patient, improvement in muscle tone was correlated with plasma concentration. Plasma concentrations were variable from patient to patient at a given dose. Although 16 mg produced a larger effect, adverse events including hypotension were more common and more severe than in the 8 mg group. There were no differences in the number of spasms occurring in each group.
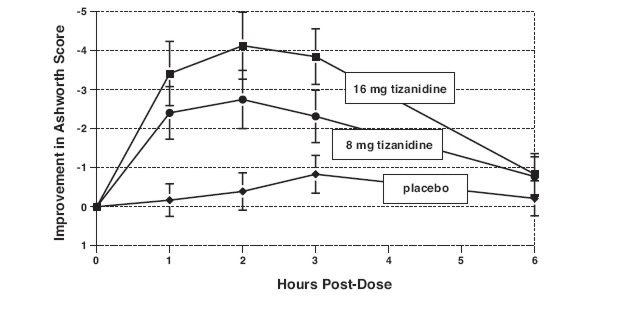
Figure 2: Single Dose Study—Mean Change in Muscle Tone from Baseline as Measured by the Ashworth Scale ± 95% Confidence Interval (A Negative Ashworth Score Signifies an Improvement in Muscle Tone from Baseline)
Seven-Week Study in Patients with Spinal Cord Injury with Spasticity
In a 7-week study (Study 2), 118 patients with spasticity secondary to spinal cord injury were randomized to either placebo or tizanidine tablets. Steps similar to those taken in the first study were employed to ensure the integrity of blinding.
Patients were titrated over 3 weeks up to a maximum tolerated dose or 36 mg daily given in three unequal doses (e.g., 10 mg given in the morning and afternoon and 16 mg given at night). Patients were then maintained on their maximally tolerated dose for 4 additional weeks (i.e., maintenance phase). Throughout the maintenance phase, muscle tone was assessed on the Ashworth scale within a period of 2.5 hours following either the morning or afternoon dose. The number of daytime spasms was recorded daily by patients.
At endpoint (the protocol-specified time of outcome assessment), there was a statistically significant reduction in muscle tone and frequency of spasms in the tizanidine tablets treated group compared to placebo. The reduction in muscle tone was not associated with a reduction in muscle strength (a desirable outcome) but also did not lead to any consistent advantage of tizanidine tablets treated patients on measures of activities of daily living. Figure 3 below shows a comparison of the mean change in muscle tone from baseline as measured by the Ashworth scale.
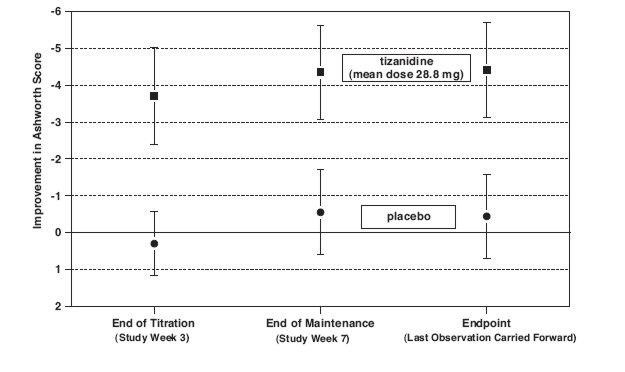
Figure 3: Seven Week Study—Mean Change in Muscle Tone 0.5–2.5 Hours After Dosing as Measured by the Ashworth Scale ± 95% Confidence Interval (A Negative Ashworth Score Signifies an Improvement in Muscle Tone from Baseline)
16 HOW SUPPLIED/STORAGE AND HANDLING
16.2 Tizanidine tablets
Tizanidine tablets, USP for oral administration are available as:
The 2 mg tablets are White to off-white, round uncoated tablets debossed with "39" on one side and functionally scored on the other side. They are supplied as:
Bottles of 30 Tablets NDC: 69844-107-01
Bottles of 150 Tablets NDC: 69844-107-02
Bottles of 1,000 Tablets NDC: 69844-107-03
The 4 mg tablets are White to off-white, round uncoated tablets with functionally scored on one side and debossed with "40'' on the other side. They are supplied as:
Bottles of 30 Tablets NDC: 69844-108-01
Bottles of 150 Tablets NDC: 69844-108-02
Bottles of 1,000 Tablets NDC: 69844-108-03
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Dispense in containers with child resistant closure.
17 PATIENT COUNSELING INFORMATION
Advise patients they should not take tizanidine tablets if they are taking fluvoxamine or ciprofloxacin because of the increased risk of serious adverse reactions including severe lowering of blood pressure and sedation. Instruct patients to inform their physicians or pharmacists when they start or stop taking any medication because of the risks associated with interaction between tizanidine tablets and other medicines.
Tizanidine tablets Dosing
Tell patients to take tizanidine tablets exactly as prescribed (consistently either with or without food) and not to switch between tablets and capsules. Inform patients that they should not take more tizanidine tablets than prescribed because of the risk of adverse events at single doses greater than 8 mg or total daily doses greater than 36 mg. Tell patients that they should not suddenly discontinue tizanidine tablets, because rebound hypertension and tachycardia may occur.
Effects of tizanidine tablets
Warn patients that they may experience hypotension and to be careful when changing from a lying or sitting to a standing position. Tell patients that tizanidine tablets may cause them to become sedated or somnolent and they should be careful when performing activities that require alertness, such as driving a vehicle or operating machinery. Tell patients that the sedation may be additive when tizanidine tablets is taken in conjunction with drugs (baclofen, benzodiazepines) or substances (e.g., alcohol) that act as CNS depressants. Remind patients that if they depend on their spasticity to sustain posture and balance in locomotion, or whenever spasticity is utilized to obtain increased function, that tizanidine tablets decreases spasticity and caution should be used.
All Brand names mentioned are registered trademarks of their respective owners and are not of Graviti Pharmaceuticals Private Limited.
Manufactured for:
Graviti Pharmaceuticals Inc.,
Wilmington, Delaware – 19801, USA.
Manufactured by:
Graviti Pharmaceuticals Pvt. Ltd.
Telangana-502307, INDIA.
Made in India
Revised: June 2024
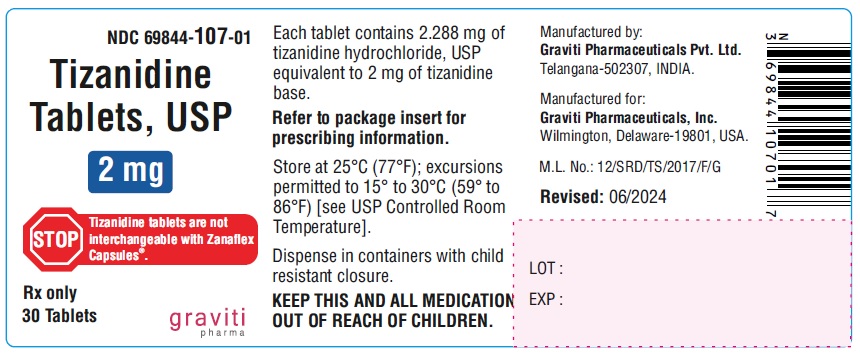
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 69844-107-01
Tizanidine Tablets, USP
2 mg*
STOP: Tizanidine tablets are not interchangeable with Tizanidine Capsules.
Rx only
30 Tablets
NDC: 69844-108-01
Tizanidine Tablets, USP
4 mg*
STOP: Tizanidine tablets are not interchangeable with Tizanidine Capsules.
Rx only
30 Tablets

| TIZANIDINE
tizanidine tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| TIZANIDINE
tizanidine tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Graviti Pharmaceuticals Private Limited (650884781) |
| Registrant - Graviti Pharmaceuticals Private Limited (650884781) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Graviti Pharmaceuticals Private Limited | 650884781 | MANUFACTURE(69844-107, 69844-108) , ANALYSIS(69844-107, 69844-108) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.