JETREA- ocriplasmin injection, solution
JETREA by
Drug Labeling and Warnings
JETREA by is a Prescription medication manufactured, distributed, or labeled by ThromboGenics Inc., Patheon UK Limited, Patheon Italia S.P.A, Fujifilm Diosynth Biotechnologies UK. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use JETREA safely and effectively. See full prescribing information for JETREA
JETREA® (ocriplasmin) injection, for intravitreal injection
Initial U.S. Approval: 2012
RECENT MAJOR CHANGES
Dosage and Administration (2) 02/2017 INDICATIONS AND USAGE
JETREA is a proteolytic enzyme indicated for the treatment of symptomatic vitreomacular adhesion. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: Single-use glass vial containing JETREA 0.375 mg in 0.3 mL solution for intravitreal injection (1.25 mg/mL). (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Decreases in vision due to progression of the condition with traction may occur requiring surgical intervention. Patients should be monitored and instructed to report any symptoms without delay. (5.1)
- Intravitreal injection procedure associated effects (intraocular inflammation/infection, intraocular hemorrhage and increased intraocular pressure (IOP)) may occur following an intravitreal injection. Patients should be monitored and instructed to report any symptoms without delay. (5.2)
- Potential for lens subluxation. (5.3)
ADVERSE REACTIONS
- The most commonly reported reactions (≥ 5%) in patients treated with JETREA were vitreous floaters, conjunctival hemorrhage, eye pain, photopsia, blurred vision, macular hole, reduced visual acuity, visual impairment, and retinal edema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact ThromboGenics Inc. at 1-855-253-7396 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 2/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Dosing
2.3 Preparation for Administration
2.4 Administration and Monitoring
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Decreased Vision
5.2 Intravitreal Injection Procedure Associated Effects
5.3 Potential for Lens Subluxation
5.4 Retinal Breaks
5.5 Dyschromatopsia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
For single-use ophthalmic intravitreal injection only. JETREA must only be administered by a qualified physician.
This formulation of JETREA does not require dilution.
2.2 Dosing
The recommended dose is 0.125 mg (0.1 mL of the solution) administered by intravitreal injection to the affected eye once as a single dose.
2.3 Preparation for Administration
To prepare JETREA for intravitreal injection, adhere to the following instructions:
-
Remove the vial (1.25 mg/mL corresponding to 0.375 mg ocriplasmin) from the freezer and allow to thaw at room temperature (within a few minutes). Use JETREA immediately after thawing. Unopened vials in the original carton protected from light can be stored up to 8 hours when stored below 77°F (25°C). Do not refreeze a vial once it has been thawed.
-
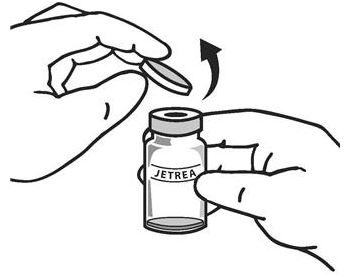
Once completely thawed, remove the protective polypropylene blue flip-off cap from the vial (see Figure 1).
-
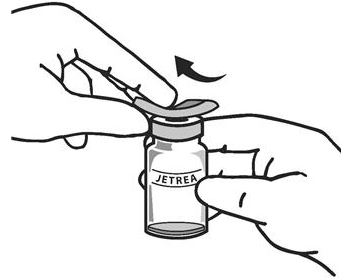
The top of the vial should be disinfected with an alcohol wipe (see Figure 2).
-
Visually inspect the vial for particulate matter. Only a clear, colorless solution without visible particles should be used.
-
Using aseptic technique, withdraw all of the solution using a sterile #19 gauge needle (slightly tilt the vial to ease withdrawal) and discard the needle after withdrawal of the vial contents (see Figure 3). Do not use this needle for the intravitreal injection.
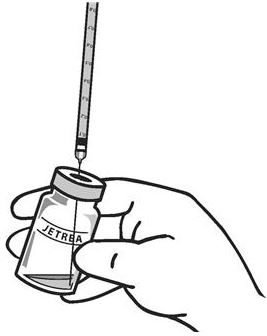
-
Replace the needle with a sterile #30 gauge needle, carefully expel the air bubbles and excess drug from the syringe and adjust the dose to the 0.1 mL mark on the syringe (corresponding to 0.125 mg ocriplasmin) (see Figure 4).
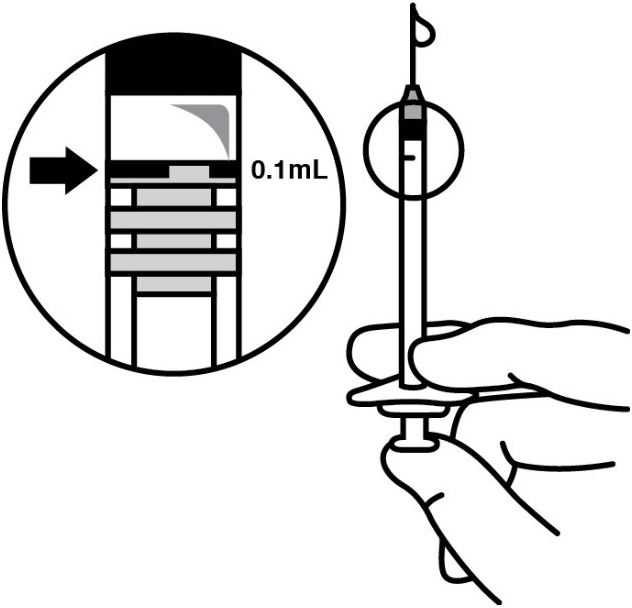
-
THE SOLUTION SHOULD BE USED IMMEDIATELY AS IT CONTAINS NO PRESERVATIVES.
-
Discard the vial and any unused portion of the solution after single use.
2.4 Administration and Monitoring
The intravitreal injection procedure should be carried out under controlled aseptic conditions, which include the use of sterile gloves, a sterile drape and a sterile eyelid speculum (or equivalent). Adequate anesthesia and a broad spectrum microbiocide should be administered according to standard medical practice.
The injection needle should be inserted 3.5 - 4.0 mm posterior to the limbus aiming towards the center of the vitreous cavity, avoiding the horizontal meridian. The injection volume of 0.1 mL is then delivered into the mid-vitreous.
Immediately following the intravitreal injection, patients should be monitored for elevation in intraocular pressure. Appropriate monitoring may consist of a check for perfusion of the optic nerve head or tonometry. If required, a sterile paracentesis needle should be available.
Following intravitreal injection, patients should be instructed to report any symptoms suggestive of endophthalmitis or retinal detachment (e.g., eye pain, redness of the eye, photophobia, blurred or decreased vision) without delay [see Patient Counseling Information (17)].
Each vial should only be used to provide a single injection for the treatment of a single eye. If the contralateral eye requires treatment, a new vial should be used and the sterile field, syringe, gloves, drapes, eyelid speculum, and injection needles should be changed before JETREA is administered to the other eye, however, treatment with JETREA in the other eye is not recommended within 7 days of the initial injection in order to monitor the post-injection course including the potential for decreased vision in the injected eye.
Repeated administration of JETREA in the same eye is not recommended [see Nonclinical Toxicology (13.2)].
After injection, any unused product must be discarded.
No special dosage modification is required for any of the populations that have been studied (e.g. gender, elderly).
-
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Decreased Vision
A decrease of ≥ 3 line of best corrected visual acuity (BCVA) was experienced by 5.6% of patients treated with JETREA and 3.2% of patients treated with vehicle in the controlled trials [see Clinical Studies (14)].
The majority of these decreases in vision were due to progression of the condition with traction and many required surgical intervention. Patients should be monitored appropriately [see Dosage And Administration (2.4)].
5.2 Intravitreal Injection Procedure Associated Effects
Intravitreal injections are associated with intraocular inflammation / infection, intraocular hemorrhage and increased intraocular pressure (IOP). In the controlled trials, intraocular inflammation occurred in 7.1% of patients injected with JETREA vs. 3.7% of patients injected with vehicle. Most of the post-injection intraocular inflammation events were mild and transient. Intraocular hemorrhage occurred in 2.4% vs. 3.7% of patients injected with JETREA vs. vehicle, respectively. Increased intraocular pressure occurred in 4.1% vs. 5.3% of patients injected with JETREA vs. vehicle, respectively.
5.3 Potential for Lens Subluxation
One case of lens subluxation was reported in a premature infant who received an intravitreal injection of 0.175 mg (1.4 times higher than the recommended dose) [see Use in Specific Populations (8.4)]. Lens subluxation was observed in three animal species (monkey, rabbit, minipig) following a single intravitreal injection that achieved vitreous concentrations of ocriplasmin 1.4 times higher than achieved with the recommended treatment dose. Administration of a second intravitreal dose in monkeys, 28 days apart, produced lens subluxation in 100% of the treated eyes [see Nonclinical Toxicology (13.2)].
5.4 Retinal Breaks
In the controlled trials, the incidence of retinal detachment was 0.9% in the JETREA group and 1.6% in the vehicle group, while the incidence of retinal tear (without detachment) was 1.1% in the JETREA group and 2.7% in the vehicle group. Most of these events occurred during or after vitrectomy in both groups. The incidence of retinal detachment that occurred pre-vitrectomy was 0.4% in the JETREA group and none in the vehicle group, while the incidence of retinal tear (without detachment) that occurred pre-vitrectomy was none in the JETREA group and 0.5% in the vehicle group.
-
6 ADVERSE REACTIONS
The following adverse reactions are described below and elsewhere in the labeling:
- Decreased Vision [see Warnings and Precautions (5.1)]
- Intravitreal Injection Procedure Associated Effects [see Warnings and Precautions (5.2) and Dosage And Administration (2.4)]
- Potential for Lens Subluxation [see Warnings and Precautions (5.3)]
- Retinal Breaks [see Warnings and Precautions (5.4) and Dosage And Administration (2.4)]
- Dyschromatopsia [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates in one clinical trial of a drug cannot be directly compared with rates in the clinical trials of the same or another drug and may not reflect the rates observed in practice.
Approximately 800 patients have been treated with an intravitreal injection of JETREA. Of these, 465 patients received an intravitreal injection of ocriplasmin 0.125 mg (187 patients received vehicle) in the 2 vehicle-controlled studies (Study 1 and Study 2).
The most common adverse reactions (incidence 5% - 20% listed in descending order of frequency) in the vehicle-controlled clinical studies were: vitreous floaters, conjunctival hemorrhage, eye pain, photopsia, blurred vision, macular hole, reduced visual acuity, visual impairment, and retinal edema.
Less common adverse reactions observed in the studies at a frequency of < 5% in patients treated with JETREA included macular edema, increased intraocular pressure, anterior chamber cell, photophobia, vitreous detachment, ocular discomfort, iritis, cataract, dry eye, metamorphopsia, pupillary reflex impaired, conjunctival hyperemia, retinal degeneration, and visual symptoms perceived in the contralateral eye.
Dyschromatopsia was reported in 2% of patients injected with JETREA, with the majority of cases reported from two uncontrolled clinical studies. In approximately half of these dyschromatopsia cases there were also electroretinographic (ERG) changes reported (a- and b-wave amplitude decrease).
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. Immunogenicity for this product has not been evaluated.
6.3 Postmarketing Experience
Night blindness has been identified during post-approval use of JETREA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C. Animal reproduction studies have not been conducted with ocriplasmin. There are no adequate and well-controlled studies of ocriplasmin in pregnant women. It is not known whether ocriplasmin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. The systemic exposure to ocriplasmin is expected to be low after intravitreal injection of a single 0.125 mg dose. Assuming 100% systemic absorption (and a plasma volume of 2700 mL), the estimated plasma concentration is 46 ng/mL. JETREA should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether ocriplasmin is excreted in human milk. Because many drugs are excreted in human milk, and because the potential for absorption and harm to infant growth and development exists, caution should be exercised when JETREA is administered to a nursing woman.
8.4 Pediatric Use
The use of JETREA in pediatric patients is not recommended. A single center, randomized, placebo controlled, double masked clinical study to investigate the safety and efficacy of a single intravitreal injection of 0.175 mg ocriplasmin in pediatric subjects as an adjunct to vitrectomy was conducted in 24 eyes of 22 patients. There were no statistical or clinical differences between groups for the induction of total macular posterior vitreous detachment (PVD), any of the secondary endpoints or adverse events.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Ocriplasmin is a recombinant truncated form of human plasmin with a molecular weight of 27.2 kDa produced by recombinant DNA technology in a Pichia pastoris expression system.
JETREA is a sterile, clear and colorless solution with no preservatives in a single-use glass vial containing 0.375 mg ocriplasmin in 0.3 mL solution for intravitreal injection.
Each vial contains 0.375 mg ocriplasmin (active), 0.16 mg citric acid, 0.56 mg mannitol, 1.35 mg sodium chloride, sodium hydroxide and hydrochloric acid (for pH adjustment), and water for injection (q.s.). The pH of the solution is 3.1.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ocriplasmin has proteolytic activity against protein components of the vitreous body and the vitreoretinal interface (VRI) (e.g. laminin, fibronectin and collagen), thereby dissolving the protein matrix responsible for the vitreomacular adhesion (VMA).
12.3 Pharmacokinetics
The intravitreal pharmacokinetics of ocriplasmin were determined in a clinical study in patients scheduled for vitrectomy where 0.125 mg ocriplasmin (corresponding to an average concentration of 29 mcg ocriplasmin per mL vitreous volume [approximately 4.3 mL/eye]) was administered as a single intravitreal dose at different time points prior to vitrectomy. The mean ocriplasmin activity levels decreased with time from injection to time of sampling as illustrated in Table 1, according to a second-order kinetic process. At 24 hours post-injection the levels in the vitreous were below 3% of the theoretical concentration reached immediately after injection.
Because of the small dose administered (0.125 mg), detectable levels of ocriplasmin in systemic circulation are not expected after intravitreal injection.
Table 1: Mean Ocriplasmin Activity Levels in Vitreous Samples after Intravitreal Injection of 0.125 mg Ocriplasmin
a 2 subjects below lower limit of detection, other 2 subjects at 0.88 and 0.57 mcg/mL
b Lower limit of detectionTime post-injection
(subjects)5-30min
(n=8)31-60min
(n=8)2-4hours
(n=8)24hr ± 2hr
(n=4)7days ± 1day
(n=4)Mean ± SD
Ocriplasmin levels
(mcg/mL)12 ±7.6 8.1 ±5.2 2.6 ±1.6 0.5 ±0.3a <0.27b Ocriplasmin enters the endogenous protein catabolism pathway through which it is rapidly inactivated via its interactions with protease inhibitor α2-antiplasmin or α2-macroglobulin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity, mutagenicity or reproductive and developmental toxicity studies were conducted with ocriplasmin.
13.2 Animal Toxicology and/or Pharmacology
The ocular toxicity of ocriplasmin after a single intravitreal dose has been evaluated in rabbits, monkeys and minipigs. Ocriplasmin induced an inflammatory response and transient ERG changes in rabbits and monkeys, which tended to resolve over time. Lens subluxation was observed in the 3 species at ocriplasmin concentrations in the vitreous at or above 41 mcg/mL, a concentration 1.4-fold above the intended clinical concentration in the vitreous of 29 mcg/mL. Intraocular hemorrhage was observed in rabbits and monkeys.
A second intravitreal administration of ocriplasmin (28 days apart) in monkeys at doses of 75 mcg/eye (41 mcg/mL vitreous) or 125 mcg/eye (68 mcg/mL vitreous) was associated with lens subluxation in all ocriplasmin treated eyes. Sustained increases in IOP occurred in two animals with lens subluxation. Microscopic findings in the eye included vitreous liquefaction, degeneration/disruption of the hyaloideocapsular ligament (with loss of ciliary zonular fibers), lens degeneration, mononuclear cell infiltration of the vitreous, and vacuolation of the retinal inner nuclear cell layer. These doses are 1.4-fold and 2.3-fold the intended clinical concentration in the vitreous of 29 mcg/mL, respectively.
-
14 CLINICAL STUDIES
The efficacy and safety of JETREA was demonstrated in two multicenter, randomized, double masked, vehicle-controlled, 6 month studies in patients with symptomatic vitreomacular adhesion (VMA). A total of 652 patients (JETREA 464, vehicle 188) were randomized in these 2 studies. Randomization was 2:1 (JETREA:vehicle) in Study 1 and 3:1 in Study 2.
Patients were treated with a single injection of JETREA or vehicle. In both of the studies, the proportion of patients who achieved VMA resolution at Day 28 (i.e., achieved success on the primary endpoint) was significantly higher in the ocriplasmin group compared with the vehicle group through Month 6 (Table 2 and Figure 5).
Table 2: Proportion of Patients with VMA Resolution in the Study Eye (Study 1, Study 2: Full Analysis Set)
Study 1 Study 2 Vehicle
N=107JETREA
N=219Difference
(95% CI)Vehicle
N=81JETREA
N=245Difference
(95% CI)n (%) n (%) n (%) n (%) Day 7 8 (7.5) 54 (24.7) 17.2 (9.6, 24.8) 1 (1.2) 36 (14.7) 13.5 (8.4, 18.5) Day 14 12 (11.2) 57 (26.0) 14.8 (6.5, 23.2) 1 (1.2) 44 (18.0) 16.7 (11.4, 22.1) Day 28 14 (13.1) 61 (27.9) 14.8 (6.0, 23.5) 5 (6.2) 62 (25.3) 19.1 (11.6, 26.7) Month 3 16 (15.0) 58 (26.5) 11.5 (2.6, 20.5) 7 (8.6) 62 (25.3) 16.7 (8.5, 24.9) Month 6 15 (14.0) 60 (27.4) 13.4 (4.5, 22.2) 10 (12.3) 65 (26.5) 14.2 (5.1, 23.2) Figure 5: Proportion of Patients with VMA Resolution in the Study Eye (Study 1 and Study 2)
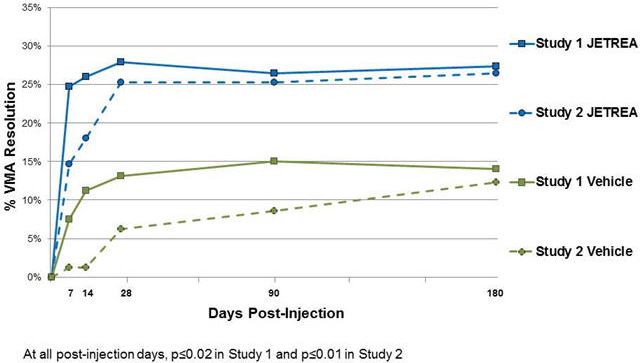
Total posterior vitreous detachment (PVD) induction in symptomatic vitreomacular adhesion patients was evaluated by B-scan ultrasound. A statistically significantly higher percentage of JETREA treated patients achieved total PVD at Day 28 compared to vehicle treated patients in Study 1 (16% vs. 6%; p=0.014) and in Study 2 (11% vs. 0%; p<0.01).
The number of patients with at least 3 lines increase in visual acuity was numerically higher in the ocriplasmin group compared to vehicle in both trials, however, the number of patients with at least a 3 lines decrease in visual acuity was also higher in the ocriplasmin group in one of the studies (Table 3 and Figure 6).
Table 3: Categorical Change from Baseline in BCVA at Month 6, Irrespective of Vitrectomy (Study 1 and Study 2)
Study 1 Study 2 JETREA Vehicle Difference JETREA Vehicle Difference N=219 N=107 (95% CI) N=245 N=81 (95% CI) ≥3 line Improvement in BCVA Month 6 28 (12.8%) 9 (8.4%) 4.4 (-2.5, 11.2) 29 (11.8%) 3 (3.8%) 8.1 (2.3, 13.9) >3 line Worsening in BCVA Month 6 16 (7.3%) 2 (1.9%) 5.4 (1.1, 9.7) 10 (4.1%) 4 (5.0%) -0.9 (-6.3, 4.5)
Figure 6: Percentage of Patients with Gain or Loss of ≥ 3 Lines of BCVA at Protocol-Specified Visits
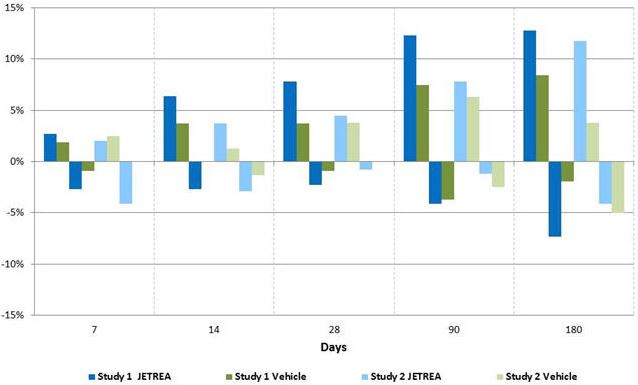
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each vial of JETREA contains 0.375 mg ocriplasmin in 0.3 mL preservative-free, citric-buffered solution (1.25 mg/mL). JETREA is supplied in a 2 mL glass vial with a blue polypropylene flip-off cap. The vial stopper is not made with natural rubber latex. Vials are for single use only.
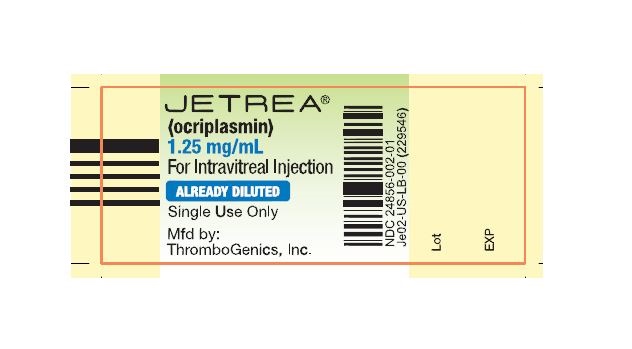
NDC: 24856-002-01
Storage
Store frozen at or below -4°F (-20°C). Protect the vials from light by storing in the original package until time of use.
-
17 PATIENT COUNSELING INFORMATION
In the days following JETREA administration, patients are at risk of developing intraocular inflammation/infection. Advise patients to seek immediate care from an ophthalmologist if the eye becomes red, sensitive to light, painful, or develops a change in vision [see Warnings and Precautions (5.2)].
Patients may experience temporary visual impairment after receiving an intravitreal injection of JETREA [see Warnings and Precautions (5.1)]. Advise patients to not drive or operate heavy machinery until this visual impairment has resolved. If visual impairment persists or decreases further, advise patients to seek care from an ophthalmologist.
Manufactured by:
ThromboGenics, Inc.
101 Wood Avenue South, Suite 610
Iselin, NJ 08830
U.S. License Number: 1866
©2012, ThromboGenics, Inc. All rights reserved.
Revised February 2017
Initial U.S. Approval: 2012
ThromboGenics U.S. patents: 7,445,775; 7,547,435; 7,914,783 and other pending patents.
- PRINCIPAL DISPLAY PANEL
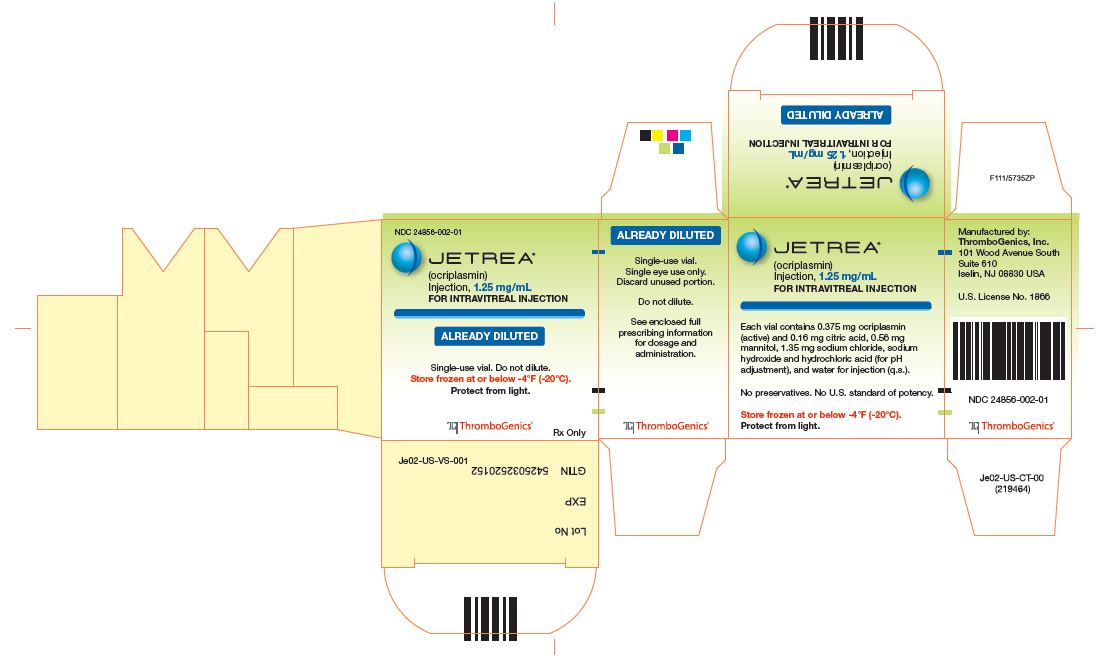
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
JETREA
ocriplasmin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24856-002 Route of Administration INTRAVITREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCRIPLASMIN (UNII: 7V6HE3DM5A) (OCRIPLASMIN - UNII:7V6HE3DM5A) OCRIPLASMIN 1.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MANNITOL (UNII: 3OWL53L36A) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24856-002-01 1 in 1 CARTON 09/18/2017 1 0.3 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125422 09/18/2017 Labeler - ThromboGenics Inc. (133263215) Establishment Name Address ID/FEI Business Operations Fujifilm Diosynth Biotechnologies UK 778997119 API MANUFACTURE(24856-002) Establishment Name Address ID/FEI Business Operations Patheon UK Limited 237710418 LABEL(24856-002) , MANUFACTURE(24856-002) , PACK(24856-002) Establishment Name Address ID/FEI Business Operations Patheon Italia S.P.A 434078638 LABEL(24856-002) , MANUFACTURE(24856-002) , PACK(24856-002)
Trademark Results [JETREA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 JETREA 85980796 4534823 Live/Registered |
Thrombogenics NV 2012-09-07 |
 JETREA 85722768 not registered Dead/Abandoned |
Thrombogenics NV 2012-09-07 |
 JETREA 79098752 4137456 Live/Registered |
Oxurion NV 2011-05-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.