REXTOVY- naloxone hydrochloride spray
Naloxone Hydrochloride Nasal by
Drug Labeling and Warnings
Naloxone Hydrochloride Nasal by is a Prescription medication manufactured, distributed, or labeled by International Medication Systems, Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use REXTOVY™ Nasal Spray safely and effectively. See full prescribing information for REXTOVY™ Nasal Spray.
REXTOVY™ (naloxone hydrochloride) Nasal Spray
Initial U.S. Approval: 1971INDICATIONS AND USAGE
REXTOVY Nasal Spray is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression for adults and pediatric patients. (1)
REXTOVY Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present. (1)
REXTOVY Nasal Spray is not a substitute for emergency medical care. (1)DOSAGE AND ADMINISTRATION
- REXTOVY Nasal Spray is for intranasal use only. (2.1)
- Seek emergency medical care immediately after use. (2.1)
- Administer one spray of REXTOVY Nasal Spray to adults or pediatric patients intranasally into one nostril. (2.2)
- Administer additional doses of REXTOVY Nasal Spray using a new nasal spray device with each dose if the patient does not respond or responds and then relapses into respiratory depression. Additional doses of REXTOVY Nasal Spray may be given every 2 to 3 minutes until emergency medical assistance arrives. (2.2)
- Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance. (2.2)
DOSAGE FORMS AND STRENGTHS
Nasal Spray: 4 mg of naloxone hydrochloride per device. Each unit-dose REXTOVY nasal spray device delivers a single spray containing 4 mg of naloxone hydrochloride (3)
CONTRAINDICATIONS
Hypersensitivity to naloxone hydrochloride or to any of the other ingredients. (4)
WARNINGS AND PRECAUTIONS
- Risk of Recurrent Respiratory and CNS Depression: Due to the duration of action of naloxone relative to the opioid, keep patient under continued surveillance and administer repeat doses of naloxone using a new nasal spray device with each dose, as necessary, while awaiting emergency medical assistance. (5.1)
- Risk of Limited Efficacy with Partial Agonists or Mixed Agonists/Antagonists: Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists, such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses may be required. (5.2)
- Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent may precipitate opioid withdrawal. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated. Monitor for the development of opioid withdrawal. (5.3)
- Risk of Cardiovascular (CV) Effects: Abrupt postoperative reversal of opioid depression may result in adverse CV effects. These events have primarily occurred in patients who had preexisting CV disorders or received other drugs that may have similar adverse CV effects. Monitor these patients closely in an appropriate healthcare setting after use of REXTOVY Nasal Spray. (5.3)
ADVERSE REACTIONS
The following adverse reactions were observed in a REXTOVY Nasal Spray clinical study: oral paraesthesia (3.7%), headache (3.7%) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Amphastar Pharmaceuticals, Inc. at 1-800-423-4136 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and PATIENT COUNSELING INFORMATION.
Revised: 5/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosing in Adults and Pediatric Patients
2.3 Dosing Modifications due to Partial Agonists or Mixed Agonist/Antagonists
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Recurrent Respiratory and Central Nervous System Depression
5.2 Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
5.3 Precipitation of Severe Opioid Withdrawal
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
REXTOVY Nasal Spray is indicated for emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression, for adult and pediatric patients.
REXTOVY Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present.
REXTOVY Nasal Spray is not a substitute for emergency medical care.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- REXTOVY Nasal Spray is for intranasal use only.
- The device is ready to use. Do not prime or test prior to administration.
- Do not attempt to reuse REXTOVY Nasal Spray. Each unit-dose device contains a single dose of naloxone and cannot be reused.
Figure 1 REXTOVY Nasal Spray Device
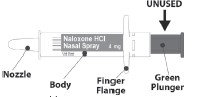
- Because treatment of suspected opioid overdose must be performed by someone other than the patient, instruct the prescription recipient to inform those around them about the presence of REXTOVY Nasal Spray and the Instructions for Use.
- Instruct the users or caregiver to read the Instructions for Use at the time they receive a prescription for REXTOVY Nasal Spray. Emphasize the following instructions to the patient or caregiver:
- Administer REXTOVY Nasal Spray as quickly as possible because prolonged respiratory depression may result in damage to the central nervous system or death.
- Always seek immediate emergency medical assistance after the first dose of REXTOVY Nasal Spray has been administered in the event of a suspected, potentially life-threatening opioid emergency because the duration of action of most opioids exceeds that of naloxone hydrochloride. Keep the patient under continued surveillance and administer repeated doses of REXTOVY Nasal Spray, as necessary, until emergency personnel arrive [see Warnings and Precautions (5.1)].
- Administer REXTOVY Nasal Spray according to the printed instructions on the carton and the Instructions for Use.
◦ Place the patient in the supine position. Prior to administration, be sure the device nozzle is inserted in either nostril of the patient and provide support to the back of the neck to allow the head to tilt back. Do not prime or test the device prior to administration.
◦To administer the dose, press firmly on the green plunger of the device and remove the REXTOVY Nasal Spray nozzle from the nostril after use.
◦If the patient responds by waking up to the voice or touch or starts breathing normally, place the person on their side (recovery position) as shown in the Instructions for Use and call for emergency medical assistance immediately after the first dose of REXTOVY Nasal Spray.◦Administer additional doses of REXTOVY Nasal Spray, using a new REXTOVY Nasal Spray, every 2 to 3 minutes as needed if the patient does not respond or responds and then relapses into respiratory depression. Administer REXTOVY Nasal Spray in alternate nostrils with each dose [see Dosing and Administration (2.2)].
2.2 Dosing in Adults and Pediatric Patients
Initial Dosing
The recommended initial dose of REXTOVY Nasal Spray in adults and pediatric patients is one spray delivered by intranasal administration, which delivers 4 mg of naloxone hydrochloride.Repeat Dosing
Seek emergency medical assistance as soon as possible after administering the REXTOVY Nasal Spray. The requirement for repeat doses of REXTOVY Nasal Spray depends upon the amount, type, and route of administration of the opioid being antagonized.Administer REXTOVY Nasal Spray in alternate nostrils with each dose.
If the patient responds to REXTOVY Nasal Spray and relapses back into respiratory depression before emergency assistance arrives, administer an additional dose of REXTOVY Nasal Spray in opposite nostril using a new REXTOVY Nasal Spray device and continue surveillance of the patient.
If the desired response is not obtained after 2 minutes, administer an additional dose of REXTOVY Nasal Spray using a new REXTOVY Nasal Spray device. If there is still no response and additional doses are available, administer additional doses of REXTOVY Nasal Spray every 2 to 3 minutes using a new REXTOVY Nasal Spray device with each dose until emergency medical assistance arrives.
Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
2.3 Dosing Modifications due to Partial Agonists or Mixed Agonist/Antagonists
Reversal of respiratory depression by partial agonists or mixed agonist/antagonists, such as buprenorphine and pentazocine, may be incomplete and require higher doses of naloxone hydrochloride or repeated administration of REXTOVY Nasal Spray using a new REXTOVY Nasal Spray [see Warnings and Precautions (5.2)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Recurrent Respiratory and Central Nervous System Depression
The duration of action of most opioids may exceed that of REXTOVY Nasal Spray resulting in a return of respiratory and/or central nervous system depression after an initial improvement in symptoms. Therefore, it is necessary to seek emergency medical assistance immediately after administration of REXTOVY Nasal Spray and to keep the patient under continued surveillance. Administer additional doses of REXTOVY Nasal Spray if the patient is not adequately responding or responds and then relapses back into respiratory depression, as necessary [see Dosage and Administration (2.2)]. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
5.2 Risk of Limited Efficacy with Partial Agonists or Mixed Agonist/Antagonists
Reversal of respiratory depression by partial agonists or mixed agonist/antagonists such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses of naloxone hydrochloride may be required to antagonize buprenorphine because the latter has a long duration of action due to its slow rate of binding and subsequent slow dissociation from the opioid receptor [see Dosage and Administration (2.3)]. Buprenorphine antagonism is characterized by a gradual onset of the reversal effects and a decreased duration of action of the normally prolonged respiratory depression.
5.3 Precipitation of Severe Opioid Withdrawal
The use of REXTOVY Nasal Spray in patients who are opioid dependent may precipitate opioid withdrawal characterized by the following signs and symptoms: body aches, diarrhea, tachycardia, fever, runny nose, sneezing, piloerection, sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may include the following signs and symptoms: convulsions, excessive crying, and hyperactive reflexes. Monitor the patient for the development of the signs and symptoms of opioid withdrawal.
Abrupt postoperative reversal of opioid depression after using naloxone hydrochloride may result in nausea, vomiting, sweating, tremulousness, tachycardia, hypotension, hypertension, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. These events have primarily occurred in patients who had pre-existing cardiovascular disorders or received other drugs that may have similar adverse cardiovascular effects. After use of naloxone hydrochloride, monitor patients with pre-existing cardiac disease or patients who have received medications with potential adverse cardiovascular effects for hypotension, ventricular tachycardia or fibrillation, and pulmonary edema in an appropriate healthcare setting. It has been suggested that the pathogenesis of pulmonary edema associated with the use of naloxone hydrochloride is similar to neurogenic pulmonary edema, i.e., a centrally mediated massive catecholamine response leading to a dramatic shift of blood volume into the pulmonary vascular bed resulting in increased hydrostatic pressures.
There may be clinical settings, particularly the postpartum period in neonates with known or suspected exposure to maternal opioid use, where it is preferable to avoid the abrupt precipitation of opioid withdrawal symptoms. In these settings, consider use of an alternative, naloxone-containing product that can be titrated to effect and, where applicable, dosed according to weight [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Recurrent Respiratory and Central Nervous System Depression [see Warnings and Precautions (5.1)]
- Precipitation of Severe Opioid Withdrawal [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In two clinical studies, N002-CL-A3 (Study A3) and N002-CL-A4 (Study A4), which comprised a total of 141 study treatments from 60 subjects, including 55 treatments using 4 mg and 10 mg of REXTOVY nasal spray (IN), the following adverse reactions were observed: oral paraesthesia (3.7%), headache (3.7%).
The following adverse reactions have been observed with other naloxone products: increased blood pressure, constipation, toothache, muscle spasms, musculoskeletal pain, headache, nasal dryness, nasal edema, nasal congestion, nasal inflammation, rhinalgia, xeroderma, abdominal pain, asthenia, dizziness, nasal discomfort, and presyncope.
6.2 Postmarketing Experience
The following adverse events have been identified during the post-approval use of naloxone hydrochloride injection in the postoperative setting. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone hydrochloride in postoperative patients have resulted in significant reversal of analgesia and have caused agitation.
Abrupt reversal of opioid effects in persons who were physically dependent on opioids has precipitated an acute withdrawal syndrome. Signs and symptoms have included: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, tachycardia. In some patients, there was aggressive behavior upon abrupt reversal of an opioid overdose. In the neonate, opioid withdrawal signs and symptoms also included convulsions, excessive crying, and hyperactive reflexes [see Warnings and Precautions (5.3)].
The following most frequently reported events (in decreasing frequency) have been identified primarily during postapproval use of naloxone hydrochloride (all routes of administration): withdrawal syndrome, vomiting, nonresponsiveness to stimuli, drug ineffective, agitation, somnolence, and loss of consciousness.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Life-sustaining therapy for opioid overdose should not be withheld (see Clinical Considerations). There is an absence of data on naloxone hydrochloride administered for known or suspected opioid overdose in pregnant patients. Available data from retrospective cohort studies on oral naloxone use in pregnant women for opioid use disorder have not identified a drug-associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes. In animal reproduction studies, no embryotoxic or teratogenic effects were observed in mice and rats treated with naloxone hydrochloride during the period of organogenesis at doses equivalent to 6-times and 12-times, respectively, a human dose of 8 mg/day (two REXTOVY Nasal Sprays) based on body surface area comparison (see Data).The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
An opioid overdose is a medical emergency and can be fatal for the pregnant woman and fetus if left untreated. Treatment with REXTOVY Nasal Spray for opioid overdose should not be withheld because of potential concerns regarding the effects of REXTOVY Nasal Spray on the fetus.Data
Animal Data
Naloxone hydrochloride was administered during organogenesis to mice and rats at doses 6-times and 12-times, respectively, a human dose of 8 mg (two REXTOVY Nasal Sprays) based on body surface area comparison. These studies demonstrated no embryotoxic or teratogenic effects due to naloxone hydrochloride.8.2 Lactation
Risk Summary
Naloxone hydrochloride is minimally orally bioavailable and is unlikely to affect the breastfed infant. There is no information regarding the presence of naloxone in human milk, or the effects of naloxone on the breastfed infant or on milk production. Published studies in lactating mothers have shown that naloxone does not affect prolactin or oxytocin hormone levels.8.4 Pediatric Use
The safety and effectiveness of REXTOVY Nasal Spray have been established in pediatric patients for known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. Use of naloxone hydrochloride in all pediatric patients is supported by adult bioequivalence studies coupled with evidence from the safe and effective use of other naloxone hydrochloride drug products. No pediatric studies were conducted for REXTOVY Nasal Spray.
Absorption of naloxone hydrochloride following intranasal administration in pediatric patients may be erratic or delayed. Even when the opiate-intoxicated pediatric patient responds appropriately to naloxone hydrochloride, he/she must be carefully monitored for at least 24 hours, as a relapse may occur as naloxone hydrochloride is metabolized.
In opioid-dependent pediatric patients, (including neonates), administration of naloxone hydrochloride may result in an abrupt and complete reversal of opioid effects, precipitating an acute opioid withdrawal syndrome. There may be clinical settings, particularly the postpartum period in neonates with known or suspected exposure to maternal opioid use, where it is preferable to avoid the abrupt precipitation of opioid withdrawal symptoms. Unlike acute opioid withdrawal in adults, acute opioid withdrawal in neonates manifesting in seizures may be life-threatening if not recognized and properly treated. Other signs and symptoms in neonates may include excessive crying and hyperactive reflexes. In these settings where it may be preferable to avoid the abrupt precipitation of acute opioid withdrawal symptoms, consider use of an alternative, naloxone hydrochloride product that can be dosed according to weight and titrated to effect [see Warnings and Precautions (5.3)].
Also, in situations where the primary concern is for infants at risk for opioid overdose, consider whether the availability of alternate naloxone-containing products may be better suited than REXTOVY Nasal Spray.
8.5 Geriatric Use
Clinical studies of REXTOVY Nasal Spray did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Geriatric patients have a greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. Therefore, the systemic exposure of naloxone hydrochloride can be higher in these patients.
-
11 DESCRIPTION
Naloxone hydrochloride is an opioid antagonist. It is chemically identified as 17-allyl-4,5α-epoxy-3,14-dihydroxymorphinan-6-one hydrochloride dihydrate. Its molecular formula is C19H21NO4 HCl 2H2O and has a molecular weight of 399.87 g/mol. It has the following structural formula:
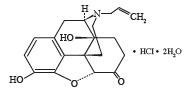
Naloxone hydrochloride dihydrate occurs as a white to slightly off-white powder. It is soluble in water, in dilute acids, and in strong alkali; slightly soluble in alcohol; practically insoluble in ether and in chloroform.
REXTOVY Nasal Spray is a prefilled unit-dose intranasal spray. Naloxone hydrochloride is contained as a solution in a stoppered glass vial within the nasal spray device. Each REXTOVY device delivers a single spray containing 4 mg of naloxone hydrochloride (equivalent to 3.6 mg of naloxone) in 0.25 mL of aqueous solution with a pH of 3.5 to 5.0.
Inactive ingredients include sodium chloride, sodium hydroxide to adjust pH, and water for injection USP.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Naloxone hydrochloride is an opioid antagonist that antagonizes opioid effects by competing for the same receptor sites.
Naloxone hydrochloride reverses the effects of opioids, including respiratory depression, sedation, and hypotension. It can also reverse the psychotomimetic and dysphoric effects of agonist-antagonists such as pentazocine.
12.2 Pharmacodynamics
When naloxone hydrochloride is administered intravenously, the onset of action is generally apparent within two minutes. The time to onset of action is shorter for intravenous compared to subcutaneous or intramuscular routes of administration. The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride.
12.3 Pharmacokinetics
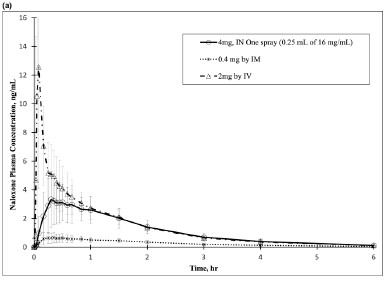
In a pharmacokinetic study on healthy adult subjects, the relative bioavailability (BA) of one nasal spray in one nostril (4 mg total dose, 0.25 mL of 16 mg/mL naloxone hydrochloride solution) was compared to a single dose of 0.4 mg naloxone hydrochloride intramuscular (IM) injection, and 2 mg naloxone hydrochloride intravenous (IV) infusion.
Absorption
The pharmacokinetics parameters obtained in the study are shown in Table 1. The pharmacokinetic curves (0-6 hours and 0-30 minutes, respectively) for REXTOVY Nasal Spray 4 mg by IN and naloxone HCl 0.4 mg delivered by IM and 2 mg naloxone by IV are provided in Figure 2, respectively.Table 1. Mean Pharmacokinetic Parameters for Naloxone Following REXTOVY Nasal Spray, IM Injection and IV Infusion of Naloxone HCl in Healthy Subjects Parameters 4 mg REXTOVY
Nasal Spray
0.4 mg Naloxone HCl
Intramuscular Injection
2 mg Naloxone HCl
Intravenous Infusion
Population N=25 N=27 N=26 AUC0-6h (ng*hr/mL ) 6.41 ± 1.33 1.54 ± 1.27 8.44 ± 1.34 AUC0-inf (ng*hr/mL ) 6.63 ± 1.32 1.60 ± 1.27 8.64 ± 1.33 Cmax (ng/mL) 3.71 ± 1.55 0.73 ± 1.56 11.10 ± 2.15 tmax (min) 32.1 ± 1.6 24.4 ± 2.4 4.9 ± 1.4 t½ (min) 67.6 ± 1.2 75.7 ± 1.2 66.2 ± 1.2 Dose-Normalized Relative
BA (%) vs. IV
40.7 ± 17.8 95.9 ± 28.5 --- Figure 2 Mean Plasma Concentration of Naloxone, (a) 0-6 hrs and (b) 0-1 hour Following Intranasal Administration of REXTOVY Nasal Spray (4mg) and IM Injection of Naloxone HCl (0.4mg)
(a)
(b)
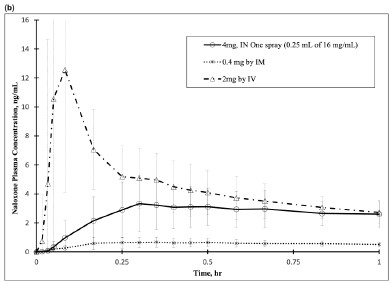
The dose-normalized relative bioavailability of one (4 mg) dose of REXTOVY Nasal Spray as compared to the 0.4 mg dose of naloxone hydrochloride administered by IV was 40.7%.
Distribution
Following parenteral administration, naloxone hydrochloride is distributed in the body and readily crosses the placenta. Plasma protein binding occurs but is relatively weak. Plasma albumin is the major binding constituent, but significant binding of naloxone hydrochloride also occurs to plasma constituents other than albumin. It is not known whether naloxone is excreted into human milk.Elimination
Following a single intranasal administration of REXTOVY Nasal Spray (4 mg dose of naloxone hydrochloride), the mean plasma half-life of naloxone hydrochloride in healthy adults was approximately 67.6 minutes, which was shorter than that observed after administrations of a 0.4 mg naloxone hydrochloride IM injection, where the half-life was 75.7 minutes. The half-life of 2 mg naloxone hydrochloride IV infusion is approximately 66.2 minutes.Metabolism
Naloxone hydrochloride is metabolized in the liver, primarily by glucuronide conjugation, with naloxone-3-glucuronide as the major metabolite.Excretion
Naloxone is excreted mainly as metabolites in urine. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of naloxone have not been completed.Mutagenesis
Naloxone was weakly positive in the Ames mutagenicity and in the in vitro human lymphocyte chromosome aberration test but was negative in the in vitro Chinese hamster V79 cell HGPRT mutagenicity assay and in the in vivo rat bone marrow chromosome aberration study.Impairment of Fertility
Reproduction studies conducted in mice and rats at doses 6-times and 12-times, respectively, a human dose of 8 mg/day (two REXTOVY Nasal Sprays) based on body surface area comparison, demonstrated no adverse effects on fertility of naloxone hydrochloride. -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Each carton contains two unit-dose REXTOVY Nasal Spray devices. Each device delivers a single spray containing 4 mg of naloxone hydrochloride.
One carton containing two REXTOVY Nasal Spray devices: NDC: 76329-3669-2
REXTOVY Nasal Spray is not made with natural rubber latex.
16.2 Storage and Handling
Store REXTOVY Nasal Spray in the blister and cartons provided.
Store at 20°C to 25°C (68°F to 77°F); excursions permitted from 4°C to 40°C (39°F to 104°F). Do not freeze. Protect from light.
Naloxone Hydrochloride freezes at temperatures below -15°C (5°F). If this happens, the device will not spray. If Naloxone Hydrochloride is frozen and is needed in an emergency, do NOT wait for Naloxone Hydrochloride to thaw. Get emergency medical help right away. However, Naloxone Hydrochloride may be thawed by allowing it to sit at room temperature for 15 minutes, and it may still be used if it has been thawed after being previously frozen.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and family members or caregivers to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Recognition of Opioid Overdose
Inform patients and their family members or caregivers about how to recognize the signs and symptoms of an opioid overdose such as the following:- Extreme somnolence -inability to awaken a patient verbally or upon a firm sternal rub.
- Respiratory depression -this can range from slow or shallow respiration to no respiration in a patient who is unarousable.
- Other signs and symptoms that may accompany somnolence and respiratory depression include the following:
◦Miosis
◦Bradycardia and/or hypotension.
Risk of Recurrent Respiratory and Central Nervous System Depression
Instruct patients and their family members or caregivers that, since the duration of action of most opioids may exceed that of REXTOVY Nasal Spray, they must seek immediate emergency medical assistance after administration of REXTOVY Nasal Spray and keep the patient under continued surveillance [see Dosage and Administration (2.2), Warnings and Precautions (5.3)].Limited Efficacy for/with Partial Agonists or Mixed Agonist/Antagonists
Instruct patients and their family members or caregivers that the reversal of respiratory depression caused by partial agonists or mixed agonist/antagonists, such as buprenorphine and pentazocine, may be incomplete and may require higher doses of naloxone hydrochloride or repeated administration of REXTOVY Nasal Spray, using a new nasal spray device each time [see Dosage and Administration (2.3), Warnings and Precautions (5.2)].Precipitation of Severe Opioid Withdrawal
Instruct patients and their family members or caregivers that the use of REXTOVY Nasal Spray in patients who are opioid dependent may precipitate opioid withdrawal [see Warnings and Precautions (5.3), Adverse Reactions (6)].Administration Instructions
Instruct patients and their family members or caregivers to:- Ensure REXTOVY Nasal Spray is readily available in locations where persons may be intentionally or accidentally exposed to an opioid overdose (i.e., opioid emergencies).
- Use each REXTOVY Nasal Spray device only one time. Do not test or prime prior to use [see Dosage and Administration (2.1)].
- Administer REXTOVY Nasal Spray as quickly as possible if a patient is unresponsive and an opioid overdose is suspected, even when in doubt, because prolonged respiratory depression may result in damage to the central nervous system or death. REXTOVY Nasal Spray is not a substitute for emergency medical care [see Dosage and Administration (2.1)].
- Lay the patient on their back and administer REXTOVY Nasal Spray into one nostril while providing support to the back of the neck to allow the head to tilt back [see Dosage and Administration (2.1)].
- If the patient responds by waking up to the voice or touch or starts breathing normally, place them in the recovery position by turning them to their side as shown in the Instructions for Use and call for emergency medical assistance immediately after administration of REXTOVY Nasal Spray. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance [see Dosage and Administration (2.1)].
- Monitor the patient and re-administer additional doses of REXTOVY Nasal Spray every 2 to 3 minutes, using a new REXTOVY Nasal Spray device. If the patient is not responding or responds and then relapses back into respiratory depression, administer REXTOVY Nasal Spray in alternate nostrils with each dose [see Dosage and Administration (2.1)].
- Replace REXTOVY Nasal Spray before its expiration date.
- SPL UNCLASSIFIED SECTION
-
Instructions for UseREXTOVY (rex toe' vee)(Naloxone Hydrochloride)Nasal Spray
You and your family members or caregivers should read the Instructions for Use that comes with REXTOVY™ Nasal Spray before using it. Talk to your healthcare provider if you and your family members or caregivers have any questions about the use of REXTOVY™ Nasal Spray.
Use REXTOVY™ Nasal Spray for known or suspected opioid overdose in adults and children.
Important: For use in the nose only.
- Do not remove or test the REXTOVY™ Nasal Spray until ready to use.
- Each REXTOVY™ Nasal Spray has 1 dose and cannot be reused.
- You do not need to prime REXTOVY™ Nasal Spray.
How to use REXTOVY™ Nasal Spray:

How should I store REXTOVY™ Nasal Spray?
- Store at room temperature between 68°F to 77°F (20°C to 25°C). REXTOVY™ Nasal Spray may be stored for short periods between 39°F to 104°F (4°C to 40°C).
- Do not freeze. Do not expose to temperatures below 39°F (4°C) or above 104°F (40°C).
- Keep REXTOVY™ Nasal Spray in its box until ready to use. Protect from light.
- Replace REXTOVY™ Nasal Spray before the expiration date on the box.
Keep REXTOVY™ Nasal Spray and all medicines out of the reach of children.
Distributed by International Medication Systems, Limited, So. El Monte, CA 91733, U.S.A.
For more information, go to www.amphastar.com or call 1-800-423-4136.This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: 06/2023
7036690F/6-23
-
PATIENT INFORMATIONREXTOVY (rex toe' vee)(Naloxone Hydrochloride)Nasal Spray
You and your family members or caregivers should read this Patient Information leaflet before an opioid emergency happens. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about REXTOVY™ Nasal Spray?
REXTOVY™ Nasal Spray is used to temporarily reverse the effects of opioid medicines. The medicine in REXTOVY™ Nasal Spray has no effect in people who are not taking opioid medicines. Always carry REXTOVY™ Nasal Spray with you in case of an opioid emergency.
1. Use REXTOVY™ Nasal Spray right away if you or your caregiver think signs or symptoms of an opioid emergency are present, even if you are not sure, because an opioid emergency can cause severe injury or death. Signs and symptoms of an opioid emergency may include:
- unusual sleepiness and you are not able to awaken the person with a loud voice or by rubbing firmly on the middle of their chest (sternum)
- breathing problems including slow or shallow breathing in someone difficult to awaken or who looks like they are not breathing
- the black circle in the center of the colored part of the eye (pupil) is very small, sometimes called “pinpoint pupils,” in someone difficult to awaken
2. Family members, caregivers, or other people who may have to use REXTOVY™ Nasal Spray in an opioid emergency should know where REXTOVY™ Nasal Spray is stored and how to give REXTOVY™ Nasal Spray before an opioid emergency happens.
3. Get emergency medical help right away after giving the first dose of REXTOVY™ Nasal Spray. Rescue breathing or CPR (cardiopulmonary resuscitation) may be given while waiting for emergency medical help.
4. The signs and symptoms of an opioid emergency can return after REXTOVY™ Nasal Spray is given. If this happens, give another dose after 2 minutes, then every 2 to 3 minutes using a new REXTOVY™ Nasal Spray and watch the person closely until emergency help is received.
What is REXTOVY™ Nasal Spray?
- REXTOVY™ Nasal Spray is a prescription medicine used in adults and children for the treatment of an opioid emergency such as an overdose or a possible opioid overdose with signs of breathing problems and severe sleepiness or not being able to respond.
- REXTOVY™ Nasal Spray is to be given right away and does not take the place of emergency medical care. Get emergency medical help right away after giving the first dose of REXTOVY™ Nasal Spray, even if the person wakes up.
- REXTOVY™ Nasal Spray is safe and effective in children for known or suspected opioid overdose.
Who should not use REXTOVY™ Nasal Spray?
Do not use REXTOVY™ Nasal Spray if you are allergic to naloxone hydrochloride or any of the ingredients in REXTOVY™ Nasal Spray. See the end of this leaflet for a complete list of ingredients in REXTOVY™ Nasal Spray.
What should I tell my healthcare provider before using REXTOVY™ Nasal Spray?
Before using REXTOVY™ Nasal Spray, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems
- are pregnant or plan to become pregnant. Use of REXTOVY™ Nasal Spray may cause withdrawal symptoms in your unborn baby. Your unborn baby should be examined by a healthcare provider right away after you use REXTOVY™ Nasal Spray.
- are breastfeeding or plan to breastfeed. It is not known if REXTOVY™ Nasal Spray passes into your breast milk.
Tell your healthcare provider about the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use REXTOVY™ Nasal Spray?
Read the "Instructions for Use" at the end of this Patient Information leaflet for detailed information about the right way to use REXTOVY™ Nasal Spray.- Use REXTOVY™ Nasal Spray exactly as prescribed by your healthcare provider.
- Each REXTOVY™ Nasal Spray contains only 1 dose of medicine and cannot be reused.
- Lay the person on their back. Support their neck with your hand and allow the head to tilt back before giving REXTOVY™ Nasal Spray.
- REXTOVY™ Nasal Spray should be given into one nostril.
- Call for emergency medical assistance immediately after the first dose of REXTOVY™ Nasal Spray.
- If the person responds by waking up to the voice or touch or starts breathing normally, place the person on their side (recovery position).
- If the person does not respond or stops breathing normally and additional doses are needed, give REXTOVY™ Nasal Spray in the other nostril.
What are the possible side effects of REXTOVY™ Nasal Spray?
REXTOVY™ Nasal Spray may cause serious side effects,including:- Sudden opioid withdrawal symptoms. In someone who has been using opioids regularly, opioid withdrawal symptoms can happen suddenly after receiving REXTOVY™ Nasal Spray and may include
o body aches o sneezing o nervousness o diarrhea o goose bumps o restlessness or irritability o increased heart rate o sweating o shivering or trembling o fever o yawning o stomach cramping o runny nose o nausea or vomiting o weakness o increased blood pressure In infants under 4 weeks old who have been receiving opioids regularly, sudden opioid withdrawal may be life-threatening if not treated the right way. Signs and symptoms include: seizures, crying more than usual, and increased reflexes.
The most common side effects of REXTOVY™ Nasal Spray include tingling in your mouth, and headache.
These are not all of the possible side effects of REXTOVY™ Nasal Spray. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store REXTOVY™ Nasal Spray?
- Store at room temperature between 68°F to 77°F (20°C to 25°C). REXTOVY™ Nasal Spray may be stored for short periods between 39°F to 104°F (4°C to 40°C).
- Do not freeze. Do not expose to temperatures below 39°F (4°C) or above 104°F (40°C).
- Keep REXTOVY™ Nasal Spray in its box until ready to use. Protect from light.
- Replace REXTOVY™ Nasal Spray before the expiration date on the box.
Keep REXTOVY™ Nasal Spray and all medicines out of the reach of children.
General information about the safe and effective use of REXTOVY™ Nasal Spray.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use REXTOVY™ Nasal Spray for a condition for which it was not prescribed. You can ask your pharmacist or healthcare provider for information about REXTOVY™ Nasal Spray that is written for health professionals.What are the ingredients in REXTOVY™ Nasal Spray?
Active ingredient: naloxone hydrochloride
Inactive ingredients: sodium chloride, sodium hydroxide to adjust pH and water for injection USP
REXTOVY™ Nasal Spray is not made with natural rubber latex.Distributed by International Medication Systems, Limited, So. El Monte, CA 91733, U.S.A.
For more information, go to www.amphastar.com or call 1-800-423-4136.This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 06/2023
-
Carton Label
Principal Display Panel Text:
NDC 76329-3669-2 Rx Only
REXTOVY™
(Naloxone HCl)
Nasal Spray4 mg per Device
For use in the Nose OnlyUSE FOR KNOWN OR SUSPECTED
OPIOID OVERDOSE
Seek Emergency Medical AttentionSee Instructions for Use for
Administration.
This box contains two (2) unit-dose
4 mg NASAL Spray devices.Deliver full dose into one nostril
Store at 20˚C to 25˚C (68˚F to 77˚F);
excursions permitted from 4˚C to 40˚C
(39˚F to 104˚F).- Do not freeze
- Protect from excessive heat
- Protect from light
INTERNATIONAL MEDICATION SYSTEMS, LIMITED
So. El Monte, CA 91733, U.S.A.
An Amphastar Pharmaceuticals Company
www.Amphastar.com
5636690D/3-23 Stock No. 3669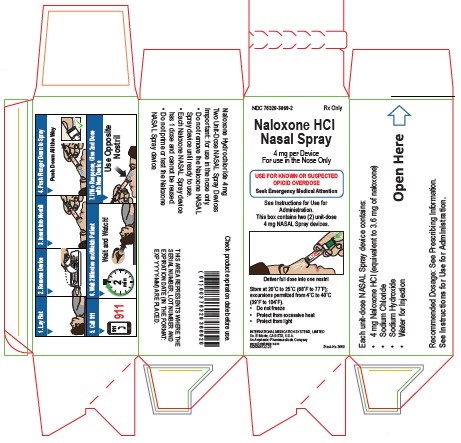
-
INGREDIENTS AND APPEARANCE
REXTOVY
naloxone hydrochloride sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 76329-3669 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.25 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76329-3669-2 2 in 1 CONTAINER 05/07/2024 1 0.25 mL in 1 CONTAINER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208969 05/07/2024 Labeler - International Medication Systems, Ltd. (055750020) Establishment Name Address ID/FEI Business Operations International Medication Systems, Ltd. 055750020 analysis(76329-3669) , manufacture(76329-3669)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.