PREV-RX- folic acid, vitamin d3, vitamin b12, vitamin e, choline, calcium tablet
Prev-Rx by
Drug Labeling and Warnings
Prev-Rx by is a Other medication manufactured, distributed, or labeled by Poly Pharmaceuticals, Inc, Formulation Technology Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
STATEMENT OF IDENTITY
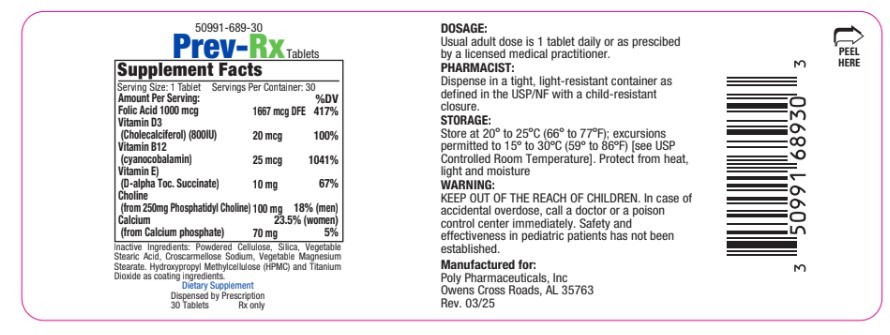
Prev-Rx is indicated for dietary management of patients with unique nutritional needs requiring increased folate, phosphatidyl choline, Vitamin D3, Vitamin B12 or Vitamin E levels or used in improving the nutritional status of patients with folic acid, phosphatidyl choline, Vitamin D3, Vitamin B12 or Vitamin E deficiency. May also be recommended for patients who are advancing in age and may benefit from supplementation to aid in mental acuity. This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent disease.
- DOSAGE & ADMINISTRATION
- HEALTH CLAIM
-
WARNINGS
KEEP OUT OF THE REACH OF CHILDREN. In case of accidental overdose, call a doctor or a poison control center immediately. Safety
and effectiveness in pediatric patients has not been established. Tell your doctor if you have: kidney problems, thyroid disease or heart disease.
This medication should only be used as directed by your doctor during pregnancy or while breastfeeding. Consult your doctor about the risk and benefits. Folic acid in doses above 0.1mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological
manifestations progress. There is evidence that the anticonvulsant action of phenytoin is antagonized by folic acid. Patients with epilepsy controlled by phenytoin may require increased dosing of phenytoin. - PRECAUTIONS
- SAFE HANDLING WARNING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PREV-RX
folic acid, vitamin d3, vitamin b12, vitamin e, choline, calcium tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:50991-689 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1000 ug CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 20 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 25 ug .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL SUCCINATE, D- 10 mg CHOLINE (UNII: N91BDP6H0X) (CHOLINE - UNII:N91BDP6H0X) CHOLINE 100 mg CALCIUM (UNII: SY7Q814VUP) (CALCIUM - UNII:SY7Q814VUP) CALCIUM 70 mg Inactive Ingredients Ingredient Name Strength POWDERED CELLULOSE (UNII: SMD1X3XO9M) SILICA (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYDROXYPROPYL METHYLCELLULOSE (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:50991-689-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 05/09/2025 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 11 mm scoring 1 Labeler - Poly Pharmaceuticals, Inc (198449894) Registrant - Poly Pharmaceuticals, Inc (198449894) Establishment Name Address ID/FEI Business Operations Formulation Technology Incorporated 062525910
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.