INDOMETHACIN injection, powder, lyophilized, for solution
INDOMETHACIN by
Drug Labeling and Warnings
INDOMETHACIN by is a Prescription medication manufactured, distributed, or labeled by Zydus Pharmaceuticals USA Inc., Navinta LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
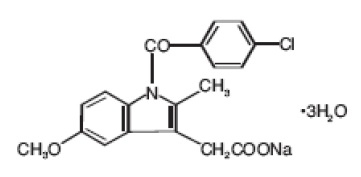
Sterile Indomethacin for injection, USP for intravenous administration is lyophilized indomethacin for injection. Each vial of indomethacin for injection contains indomethacin sodium equivalent to 1 mg indomethacin as a white to yellow lyophilized powder or plug. Variations in the size of the lyophilized plug and the intensity of color have no relationship to the quality or amount of indomethacin present in the vial. Indomethacin for injection is designated chemically as 1-(4-chlorobenzoyl)-5-methoxy-2-methyl 1H-indole-3-acetic acid, sodium salt, trihydrate. Its molecular weight is 433.82. Its empirical formula is C19H15ClNNaO43H2O and its structural formula is:
-
CLINICAL PHARMACOLOGY
Although the exact mechanism of action through which indomethacin causes closure of a patent ductus arteriosus is not known, it is believed to be through inhibition of prostaglandin synthesis. Indomethacin has been shown to be a potent inhibitor of prostaglandin synthesis, both in vitro and in vivo. In human newborns with certain congenital heart malformations, PGE 1 dilates the ductus arteriosus. In fetal and newborn lambs, E type prostaglandins have also been shown to maintain the patency of the ductus, and as in human newborns, indomethacin causes its constriction.
Studies in healthy young animals and in premature infants with patent ductus arteriosus indicated that, after the first dose of intravenous indomethacin, there was a transient reduction in cerebral blood flow velocity and cerebral blood flow. Similar decreases in mesenteric blood flow and velocity have been observed. The clinical significance of these effects has not been established.
In double-blind, placebo-controlled studies of indomethacin for injection in 460 small pre-term infants, weighing 1750 g or less, the neonates treated with placebo had a ductus closure rate after 48 hours of 25 to 30 percent, whereas those treated with indomethacin for injection had a 75 to 80 percent closure rate. In one of these studies, a multicenter study, involving 405 pre-term infants, later re opening of the ductus arteriosus occurred in 26 percent of neonates treated with indomethacin for injection, however, 70 percent of these closed subsequently without the need for surgery or additional indomethacin.
Pharmacokinetics and Metabolism:
The disposition of indomethacin following intravenous administration (0.2 mg/kg) in pre-term neonates with patent ductus arteriosus has not been extensively evaluated. Even though the plasma half-life of indomethacin was variable among premature infants, it was shown to vary inversely with postnatal age and weight. In one study, of 28 neonates who could be evaluated, the plasma half-life in those less than 7 days old averaged 20 hours (range: 3-60 hours, n=18). In neonates older than 7 days, the mean plasma half-life of indomethacin was 12 hours (range: 4-38 hours, n=10). Grouping the neonates by weight, mean plasma half-life in those weighing less than 1000 g was 21 hours (range: 9-60 hours, n=10); in those neonates weighing more than 1000 g, the mean plasma half-life was 15 hours (range: 3-52 hours, n=18).
Following intravenous administration in adults, indomethacin is eliminated via renal excretion, metabolism, and biliary excretion. Indomethacin undergoes appreciable enterohepatic circulation. The mean plasma half-life of indomethacin is 4.5 hours. In the absence of enterohepatic circulation, it is 90 minutes. Indomethacin has been found to cross the blood-brain barrier and the placenta.
In adults, about 99 percent of indomethacin is bound to protein in plasma over the expected range of therapeutic plasma concentrations. The percent bound in neonates has not been studied. In controlled trials in premature infants, however, no evidence of bilirubin displacement has been observed as evidenced by increased incidence of bilirubin encephalopathy (kernicterus).
-
INDICATIONS AND USAGE
Indomethacin for injection is indicated to close a hemodynamically significant patent ductus arteriosus in premature infants weighing between 500 and 1750 g when after 48 hours usual medical management (e.g., fluid restriction, diuretics, digitalis, respiratory support, etc.) is ineffective. Clear-cut clinical evidence of a hemodynamically significant patent ductus arteriosus should be present, such as respiratory distress, a continuous murmur, a hyperactive precordium, cardiomegaly and pulmonary plethora on chest x-ray.
-
CONTRAINDICATIONS
Indomethacin for injection is contraindicated in: neonates with proven or suspected infection that is untreated; neonates who are bleeding, especially those with active intracranial hemorrhage or gastrointestinal bleeding; neonates with thrombocytopenia; neonates with coagulation defects; neonates with or who are suspected of having necrotizing enterocolitis; neonates with significant impairment of renal function; neonates with congenital heart disease in whom patency of the ductus arteriosus is necessary for satisfactory pulmonary or systemic blood flow (e.g., pulmonary atresia, severe tetralogy of Fallot, severe coarctation of the aorta).
-
WARNINGS
Gastrointestinal Effects:
In the collaborative study, major gastrointestinal bleeding was no more common in those neonates receiving indomethacin than in those neonates on placebo. However, minor gastrointestinal bleeding (i.e., chemical detection of blood in the stool) was more commonly noted in those neonates treated with indomethacin. Severe gastrointestinal effects have been reported in adults with various arthritic disorders treated chronically with oral indomethacin. [For further information, see package insert for Indomethacin Capsules]
Central Nervous System Effects:
Prematurity per se, is associated with an increased incidence of spontaneous intraventricular hemorrhage. Because indomethacin may inhibit platelet aggregation, the potential for intraventricular bleeding may be increased. However, in the large multicenter study of indomethacin for injection (see CLINICAL PHARMACOLOGY), the incidence of intraventricular hemorrhage in neonates treated with indomethacin for injection was not significantly higher than in the control neonates.
Renal Effects:
Indomethacin for injection may cause significant reduction in urine output (50 percent or more) with concomitant elevations of blood urea nitrogen and creatinine, and reductions in glomerular filtration rate and creatinine clearance. These effects in most neonates are transient, disappearing with cessation of therapy with indomethacin for injection. However, because adequate renal function can depend upon renal prostaglandin synthesis, indomethacin for injection may precipitate renal insufficiency, including acute renal failure, especially in neonates with other conditions that may adversely affect renal function (e.g., extracellular volume depletion from any cause, congestive heart failure, sepsis, concomitant use of any nephrotoxic drug, hepatic dysfunction). When significant suppression of urine volume occurs after a dose of indomethacin for injection, no additional dose should be given until the urine output returns to normal levels.
Indomethacin for injection in pre-term infants may suppress water excretion to a greater extent than sodium excretion. When this occurs, a significant reduction in serum sodium values (i.e., hyponatremia) may result. Neonates should have serum electrolyte determinations done during therapy with indomethacin for injection. Renal function and serum electrolytes should be monitored (see PRECAUTIONS, Drug Interactions and DOSAGE AND ADMINISTRATION).
-
PRECAUTIONS
General:
Indomethacin may mask the usual signs and symptoms of infection. Therefore, the physician must be continually on the alert for this and should use the drug with extra care in the presence of existing controlled infection.
Severe hepatic reactions have been reported in adults treated chronically with oral indomethacin for arthritic disorders. [For further information, see package insert for Indomethacin Capsules] If clinical signs and symptoms consistent with liver disease develop in the neonate, or if systemic manifestations occur, indomethacin for injection should be discontinued.
Indomethacin for injection may inhibit platelet aggregation. In one small study, platelet aggregation was grossly abnormal after indomethacin therapy (given orally to premature infants to close the ductus arteriosus). Platelet aggregation returned to normal by the tenth day. Premature infants should be observed for signs of bleeding.
The drug should be administered carefully to avoid extravascular injection or leakage as the solution may be irritating to tissue.
Drug Interactions:
Since renal function may be reduced by indomethacin for injection, consideration should be given to reduction in dosage of those medications that rely on adequate renal function for their elimination. Because the half-life of digitalis (given frequently to pre-term infants with patent ductus arteriosus and associated cardiac failure) may be prolonged when given concomitantly with indomethacin, the neonate should be observed closely; frequent ECGs and serum digitalis levels may be required to prevent or detect digitalis toxicity early. Furthermore, in one study of premature infants treated with indomethacin for injection and also receiving either gentamicin or amikacin, both peak and trough levels of these aminoglycosides were significantly elevated.
Therapy with indomethacin may blunt the natriuretic effect of furosemide. This response has been attributed to inhibition of prostaglandin synthesis by non-steroidal anti-inflammatory drugs. In a study of 19 premature infants with patent ductus arteriosus treated with either indomethacin for injection alone or a combination of indomethacin for injection and furosemide, results showed that neonates receiving both indomethacin for injection and furosemide had significantly higher urinary output, higher levels of sodium and chloride excretion, and higher glomerular filtration rates than did those receiving indomethacin for injection alone. In this study, the data suggested that therapy with furosemide helped to maintain renal function in the premature infant when indomethacin for injection was added to the treatment of patent ductus arteriosus.
Indomethacin usually does not influence the hypoprothrombinemia produced by anticoagulants. When indomethacin is added to anticoagulants, prothrombin time should be monitored closely. In post marketing experience, bleeding has been reported in patients on concomitant treatment with anticoagulants and indomethacin for injection. Caution should be exercised when indomethacin for injection and anticoagulants are administered concomitantly.
In some patients with compromised renal function, the co-administration of an NSAID and an ACE inhibitor or angiotensin II antagonist may result in further deterioration of renal function, including possible acute renal failure, which is usually reversible.
Neonatal Effects:
In rats and mice, oral indomethacin 4.0 mg/kg/day given during the last three days of gestation caused a decrease in maternal weight gain and some maternal and fetal deaths. An increased incidence of neuronal necrosis in the diencephalon in the live-born fetuses was observed. At 2.0 mg/kg/day, no increase in neuronal necrosis was observed as compared to the control groups. Administration of 0.5 or 4.0 mg/kg/day during the first three days of life did not cause an increase in neuronal necrosis at either dose level.
Pregnant rats, given 2.0 mg/kg/day and 4.0 mg/kg/day during the last trimester of gestation, delivered offspring whose pulmonary blood vessels were both reduced in number and excessively muscularized. These findings are similar to those observed in the syndrome of persistent pulmonary hypertension of the neonate.
-
ADVERSE REACTIONS
In a double-blind, placebo-controlled trial of 405 premature infants weighing less than or equal to 1750 g with evidence of large ductal shunting, in those neonates treated with indomethacin (n=206), there was a statistically significantly greater incidence of bleeding problems, including gross or microscopic bleeding into the gastrointestinal tract, oozing from the skin after needle stick, pulmonary hemorrhage, and disseminated intravascular coagulopathy. There was no statistically significant difference between treatment groups with reference to intracranial hemorrhage.
The neonates treated with indomethacin for injection also had a significantly higher incidence of transient oliguria and elevations of serum creatinine (greater than or equal to 1.8 mg/dL) than did the neonates treated with placebo.
The incidences of retrolental fibroplasia (grades III and IV) and pneumothorax in neonates treated with indomethacin for injection were no greater than in placebo controls and were statistically significantly lower than in surgically-treated neonates.
The following additional adverse reactions in neonates have been reported from the collaborative study, anecdotal case reports, from other studies using rectal, oral, or intravenous indomethacin for treatment of patent ductus arteriosus or in marketed use. The rates are calculated from a database which contains experience of 849 indomethacin-treated neonates reported in the medical literature, regardless of the route of administration. One year follow-up is available on 175 neonates and shows no long-term sequelae which could be attributed to indomethacin. In controlled clinical studies, only electrolyte imbalance and renal dysfunction (of the reactions listed below) occurred statistically significantly more frequently after indomethacin for injection than after placebo. Reactions marked with a single asterisk (*) occurred in 3-9 percent of indomethacin-treated neonates; those marked with a double asterisk (**) occurred in 3-9 percent of both indomethacin- and placebo-treated neonates. Unmarked reactions occurred in less than 3 percent of neonates.
Renal: renal failure, renal dysfunction in 41 percent of neonates, including one or more of the following: reduced urinary output; reduced urine sodium, chloride, or potassium, urine osmolality, free water clearance, or glomerular filtration rate; elevated serum creatinine or BUN; uremia.
Cardiovascular: intracranial bleeding**, pulmonary hypertension.
Gastrointestinal: gastrointestinal bleeding*, vomiting, abdominal distention, transient ileus, gastric perforation, localized perforation(s) of the small and/or large intestine, necrotizing enterocolitis.
Metabolic: hyponatremia*, elevated serum potassium*, reduction in blood sugar, including hypoglycemia, increased weight gain (fluid retention).
Coagulation: decreased platelet aggregation (see PRECAUTIONS).
The following adverse reactions have also been reported in neonates treated with indomethacin, however, a causal relationship to therapy with indomethacin for injection has not been established:
Cardiovascular: bradycardia.
Respiratory: apnea, exacerbation of pre-existing pulmonary infection.
Metabolic: acidosis/alkalosis.
Hematologic: disseminated intravascular coagulation, thrombocytopenia.
Ophthalmic: retrolental fibroplasia.**
A variety of additional adverse experiences have been reported in adults treated with oral indomethacin for moderate to severe rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, acute painful shoulder and acute gouty arthritis (see package insert for Indomethacin Capsules for additional information concerning adverse reactions and other cautionary statements). Their relevance to the pre-term infant receiving indomethacin for patent ductus arteriosus is unknown, however, the possibility exists that these experiences may be associated with the use of indomethacin for injection in pre-term infants.
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals USA Inc, Pennington, NJ 08534 at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
DOSAGE AND ADMINISTRATION
FOR INTRAVENOUS ADMINISTRATION ONLY.
Dosage recommendations for closure of the ductus arteriosus depend on the age of the infant at the time of therapy. A course of therapy is defined as three intravenous doses of indomethacin for injection given at 12-24 hour intervals, with careful attention to urinary output. If anuria or marked oliguria (urinary output <0.6 mL/kg/hr) is evident at the scheduled time of the second or third dose of indomethacin for injection, no additional doses should be given until laboratory studies indicate that renal function has returned to normal (see WARNINGS, Renal Effects).
Dosage according to age is as follows:
AGE at
1st dose
DOSAGE (mg/kg)
Less than
48 hours
1st
0.2
2nd
0.1
3rd
0.1
2-7 days
0.2
0.2
0.2
over
7 days
0.2
0.25
0.25
If the ductus arteriosus closes or is significantly reduced in size after an interval of 48 hours or more from completion of the first course of indomethacin for injection, no further doses are necessary. If the ductus arteriosus re-opens, a second course of 1-3 doses may be given, each dose separated by a 12-24 hour interval as described above.
If the neonate remains unresponsive to therapy with indomethacin for injection after 2 courses, surgery may be necessary for closure of the ductus arteriosus. If severe adverse reactions occur, STOP THE DRUG.
Directions For Use
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
The reconstituted solution is clear, slightly yellow and essentially free from visible particles.
The solution should be prepared only with 1 to 2 mL of preservative-free Sterile Sodium Chloride Injection, 0.9 percent or preservative-free Sterile Water for Injection. Benzyl alcohol as a preservative has been associated with toxicity in neonates. Therefore, all diluents should be preservative-free. If 1 mL of diluent is used, the concentration of indomethacin in the solution will equal approximately 0.1 mg/0.1 mL; if 2 mL of diluent are used, the concentration of the solution will equal approximately 0.05 mg/0.1 mL. Any unused portion of the solution should be discarded because there is no preservative contained in the vial. A fresh solution should be prepared just prior to each administration. Once reconstituted, the indomethacin solution may be injected intravenously. While the optimal rate of injection has not been established, published literature suggests an infusion rate over 20-30 minutes.
Indomethacin for injection is not buffered. Further dilution with intravenous infusion solutions is not recommended.
-
HOW SUPPLIED
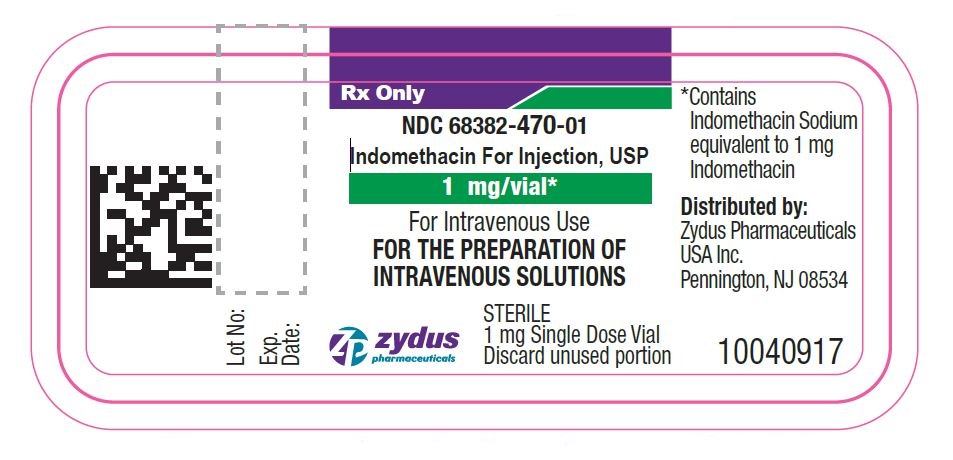
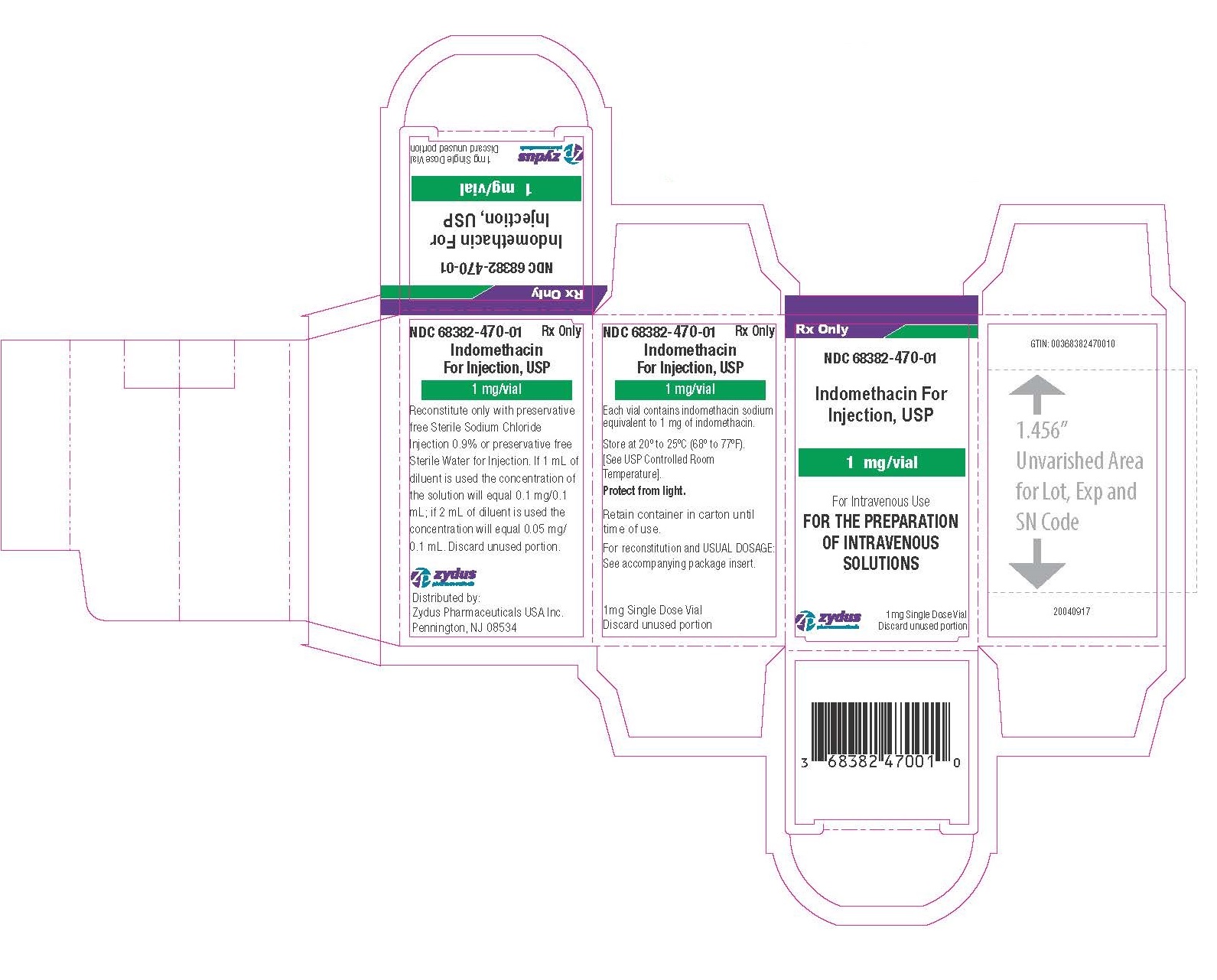
Sterile Indomethacin for injection, USP is a lyophilized white to yellow powder or plug supplied as single dose vial containing indomethacin sodium, equivalent to 1 mg indomethacin.
NDC: 68382-470-01 carton of 1mg Single Dose Vial.
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
INDOMETHACIN
indomethacin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68382-470 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength INDOMETHACIN SODIUM (UNII: 0IMX38M2GG) (INDOMETHACIN - UNII:XXE1CET956) INDOMETHACIN 1 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68382-470-01 1 in 1 CARTON 10/03/2017 1 1 in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206561 10/03/2017 Labeler - Zydus Pharmaceuticals USA Inc. (156861945) Registrant - Navinta LLC (130443810)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.