BRAVECTO- fluralaner solution
Bravecto by
Drug Labeling and Warnings
Bravecto by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SAFE HANDLING WARNING
-
DESCRIPTION
Description:
Each tube is formulated to provide a minimum dose of 18.2 mg/lb (40 mg/kg) body weight. Each milliliter contains 280 mg of fluralaner.
The chemical name of fluralaner is (±)-4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)- 4,5-dihydroisoxazol-3-yl]-2-methyl-N-[2-oxo-2-(2,2,2 - trifluoroethylamino)ethyl]benzamide. Inactive ingredients: dimethylacetamide, glycofurol, diethyltoluamide, acetone
-
VETERINARY INDICATIONS
Indications:
Bravecto kills adult fleas and is indicated for the treatment and prevention of flea infestations (Ctenocephalides felis) and the treatment and control of Ixodes scapularis (black-legged tick) infestations for 12 weeks in cats and kittens 6 months of age and older, and weighing 2.6 pounds or greater.
Bravecto is also indicated for the treatment and control of Dermacentor variabilis (American dog tick) infestations for 8 weeks in cats and kittens 6 months of age and older, and weighing 2.6 pounds or greater.
-
DOSAGE & ADMINISTRATION
Dosage and Administration:
Bravecto should be administered topically as a single dose every 12 weeks according to the Dosage Schedule below to provide a minimum dose of 18.2 mg/lb (40 mg/kg) body weight.
Bravecto may be administered every 8 weeks in case of potential exposure to Dermacentor variabilis ticks (see Effectiveness).
Dosage Schedule:
Body Weight Ranges (lb) Fluralaner content (mg/tube) Tubes Administered - * Cats over 27.5 lb should be administered the appropriate combination of tubes.
2.6 – 6.2 112.5 One >6.2 – 13.8 250 One >13.8 – 27.5* 500 One Step 1: Immediately before use, open the pouch and remove the tube. Hold the tube at the crimped end with the cap in an upright position (tip up). The cap should be rotated clockwise or counter clockwise one full turn. The cap is designed to stay on the tube for dosing and should not be removed. The tube is open and ready for application when a breaking of the seal is felt.
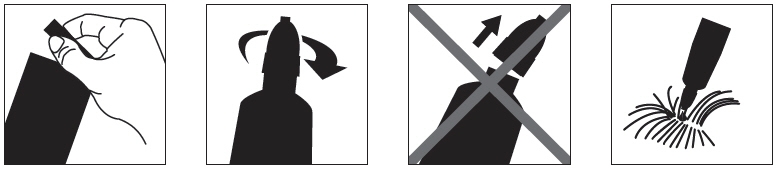
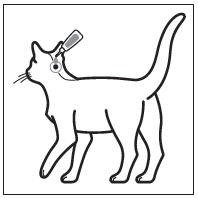
Step 2: The cat should be standing or lying with its back horizontal during application. Part the fur at the administration site. Place the tube tip vertically against the skin at the base of the skull of the cat.
Step 3: Squeeze the tube and gently apply the entire contents of Bravecto directly to the skin at the base of the skull of the cat. Avoid applying an excessive amount of solution that could cause some of the solution to run and drip off of the cat. If a second spot is needed to avoid run off, then apply the second spot slightly behind the first spot.
Treatment with Bravecto may begin at any time of the year and can continue year round without interruption.
- CONTRAINDICATIONS
-
WARNINGS
Human Warnings:
Not for human use. Keep this and all drugs out of the reach of children.
Do not contact or allow children to contact the application site until dry.
Keep the product in the original packaging until use in order to prevent children from getting direct access to the product. Do not eat, drink or smoke while handling the product. Avoid contact with skin and eyes. If contact with eyes occurs, then flush eyes slowly and gently with water. Wash hands and contacted skin thoroughly with soap and water immediately after use of the product.
The product is highly flammable. Keep away from heat, sparks, open flame or other sources of ignition.
-
PRECAUTIONS
Precautions:
For topical use only. Avoid oral ingestion. (see Animal Safety).
Use with caution in cats with a history of neurologic abnormalities. Neurologic abnormalities have been reported in cats receiving Bravecto, even in cats without a history of neurologic abnormalities (see Adverse Reactions).
Bravecto has not been shown to be effective for 12-weeks duration in kittens less than 6 months of age. Bravecto is not effective against Dermacentor variabilis ticks beyond 8 weeks after dosing (see Effectiveness).
The safety of Bravecto has not been established in breeding, pregnant and lactating cats.
-
ADVERSE REACTIONS
Adverse Reactions:
In a well-controlled U.S. field study, which included a total of 161 households and 311 treated cats (224 with fluralaner and 87 with a topical active control), there were no serious adverse reactions.
Percentage of Cats with Adverse Reactions (AR) in the Field Study Adverse Reaction
(AR)Bravecto Group:
Percent of Cats with the AR During the 105-Day Study
(n=224 cats)Control Group:
Percent of Cats with the AR During the 84-Day Study
(n=87 cats)Vomiting 7.6% 6.9% Pruritus 5.4% 11.5% Diarrhea 4.9% 1.1% Alopecia 4.9% 4.6% Decreased Appetite 3.6% 0.0% Lethargy 3.1% 2.3% Scabs/Ulcerated Lesions 2.2% 3.4% In the field study, two cats treated with fluralaner topical solution experienced ataxia. One cat became ataxic with a right head tilt 34 days after the first dose. The cat improved within one week of starting antibiotics. The ataxia and right head tilt, along with lateral recumbency, reoccurred 82 days after administration of the first dose. The cat recovered with antibiotics and was redosed with fluralaner topical solution 92 days after administration of the first dose, with no further abnormalities during the study. A second cat became ataxic 15 days after receiving its first dose and recovered the next day. The cat was redosed with fluralaner topical solution 82 days after administration of the first dose, with no further abnormalities during the study.
In a European field study, two cats from the same household experienced tremors, lethargy, and anorexia within one day of administration. The signs resolved in both cats within 48-72 hours.
In a European field study, there were three reports of facial dermatitis in humans after close contact with the application site which occurred within 4 days of application.
For technical assistance or to report a suspected adverse drug reaction, or to obtain a copy of the Safety Data Sheet (SDS), contact Merck Animal Health at 1-800-224-5318. Additional information can be found at www.bravecto.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/AnimalVeterinary/SafetyHealth.
-
CLINICAL PHARMACOLOGY
Clinical Pharmacology:
Peak fluralaner concentrations are achieved between 7 and 21 days following topical administration and the elimination halflife ranges between 11 and 13 days.
Mode of Action:
Fluralaner is for systemic use and belongs to the class of isoxazoline-substituted benzamide derivatives. Fluralaner is an inhibitor of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
Effectiveness:
In a well-controlled European laboratory study, Bravecto killed 100% of fleas 8 hours after treatment and reduced the number of live fleas on cats by > 98% within 12 hours after treatment or post-infestation for 12 weeks. In well-controlled laboratory studies, Bravecto demonstrated > 94% effectiveness against Ixodes scapularis 48 hours post- infestation for 12 weeks. Bravecto demonstrated > 98% effectiveness against Dermacentor variabilis 48 hours post-infestation for 8 weeks, but failed to demonstrate ≥ 90% effectiveness beyond 8 weeks.
In a well-controlled U.S. field study, a single dose of Bravecto reduced fleas by ≥99% for 12 weeks. Cats with signs of flea allergy dermatitis showed improvement in erythema, alopecia, papules, scales, crusts, and excoriation as a direct result of eliminating flea infestations.
-
SPL UNCLASSIFIED SECTION
Animal Safety:
Margin of Safety Study: In a margin of safety study, Bravecto was administered topically to 11- to 13-week (mean age 12 weeks)-old-kittens at 1, 3, and 5× the maximum labeled dose of 93 mg/kg at three, 8-week intervals (8 cats per group). The cats in the control group (0×) were treated with mineral oil.
There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. Cosmetic changes at the application site included matting/clumping/spiking of hair, wetness, or a greasy appearance.
Oral Safety Study: In a safety study, one dose of Bravecto topical solution was administered orally to 6- to 7-month-old-kittens at 1× the maximum labeled dose of 93 mg/kg. The kittens in the control group (0×) were administered saline orally. There were no clinically-relevant, treatment-related effects on physical examination, body weights, food consumption, clinical pathology (hematology, clinical chemistries, coagulation tests, and urinalysis), gross pathology, histopathology, or organ weights. All treated kittens experienced salivation and four of six experienced coughing immediately after administration. One treated kitten experienced vomiting 2 hours after administration.
In a well-controlled field study Bravecto was used concurrently with other medications, such as vaccines, anthelmintics, antibiotics, steroids and sedatives. No adverse reactions were observed from the concurrent use of Bravecto with other medications.
- STORAGE AND HANDLING
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 112.5 MG Tube Carton
2.6 – 6.2 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Cats and Kittens 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 112.5 MG FLURALANER
x1
NADA# 141-459
Approved by FDAMERCK
Animal Health
-
PRINCIPAL DISPLAY PANEL - 250 MG Tube Carton
>6.2 – 13.8 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Cats and Kittens 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 250 MG FLURALANER
x1
NADA# 141-459
Approved by FDAMERCK
Animal Health
-
PRINCIPAL DISPLAY PANEL - 500 MG Tube Carton
>13.8 – 27.5 lb
BRAVECTO®
(fluralaner)
Topical SolutionKILLS FLEAS and TICKS
For Cats and Kittens 6 months of age and older
CAUTION: Federal (USA) law restricts this drug to use
by or on the order of a licensed veterinarian.CONTENTS: 1 TUBE CONTAINING 500 MG FLURALANER
x1
NADA# 141-459
Approved by FDAMERCK
Animal Health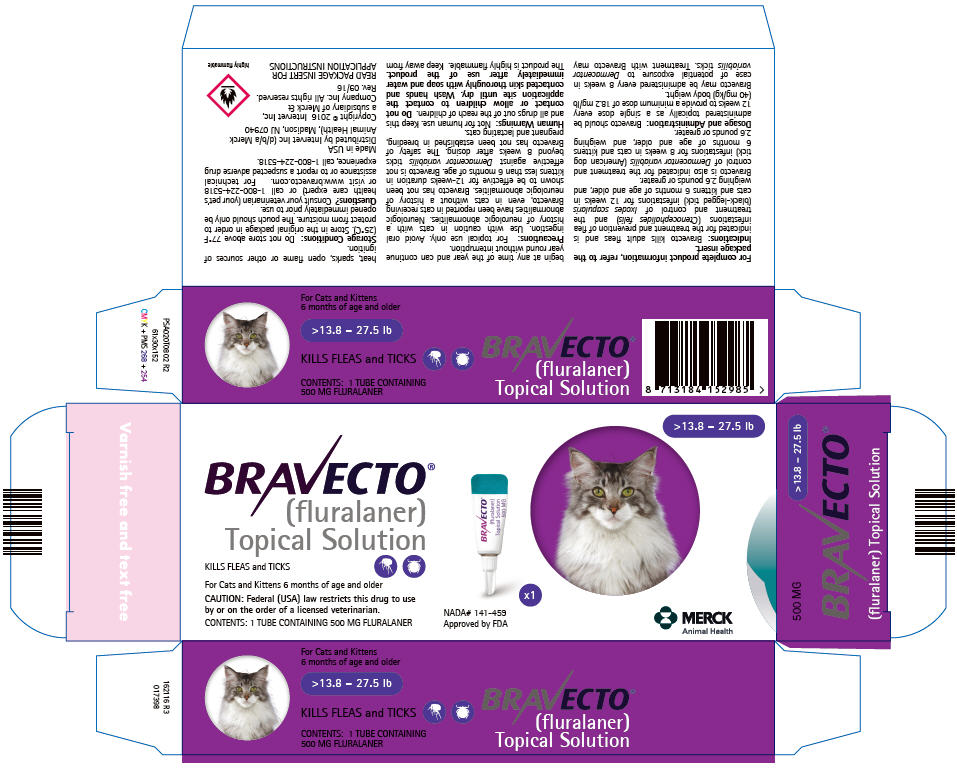
-
INGREDIENTS AND APPEARANCE
BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5505 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 112.5 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5505-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5505-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5506 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 250 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5506-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5506-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 BRAVECTO
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5507 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fluralaner (UNII: WSH8393RM5) (Fluralaner - UNII:WSH8393RM5) Fluralaner 500 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5507-02 1 in 1 CARTON 1 1 in 1 POUCH 2 NDC: 0061-5507-01 2 in 1 CARTON 2 1 in 1 POUCH Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141459 07/30/2016 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [Bravecto]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRAVECTO 97938637 not registered Live/Pending |
Intervet Inc. 2023-05-16 |
 BRAVECTO 86307345 4778693 Live/Registered |
Intervet Inc. 2014-06-11 |
 BRAVECTO 85544437 4610171 Live/Registered |
Intervet Inc. 2012-02-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.