IMITREX- sumatriptan succinate injection
IMITREX by
Drug Labeling and Warnings
IMITREX by is a Prescription medication manufactured, distributed, or labeled by GlaxoSmithKline LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use IMITREX safely and effectively. See full prescribing information for IMITREX.
IMITREX (sumatriptan succinate) injection, for subcutaneous use
Initial U.S. Approval: 1992INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- For subcutaneous use only (2.1)
- Acute treatment of migraine: single dose of 1 to 6 mg (2.1)
- Acute treatment of cluster headache: single dose of 6 mg (2.1)
- Maximum dose in a 24-hour period: 12 mg, separate doses by at least 1 hour (2.1)
- The needle shield of the prefilled syringe contains dry natural rubber (a latex derivative) which may cause allergic reactions in latex-sensitive patients (2.2)
- Patients receiving doses other than 4 or 6 mg: Use the 6-mg single-dose vial (2.3)
DOSAGE FORMS AND STRENGTHS
CONTRAINDICATIONS
- History of coronary artery disease or coronary artery vasospasm (4)
- Wolff-Parkinson-White syndrome or other cardiac accessory conduction pathway disorders (4)
- History of stroke, transient ischemic attack, or hemiplegic or basilar migraine (4)
- Peripheral vascular disease (4)
- Ischemic bowel disease (4)
- Uncontrolled hypertension (4)
- Recent (within 24 hours) use of another 5-HT1 agonist (e.g., another triptan) or of an ergotamine-containing medication (4)
- Concurrent or recent (past 2 weeks) use of monoamine oxidase-A inhibitor (4)
- Hypersensitivity to IMITREX (angioedema and anaphylaxis seen) (4)
- Severe hepatic impairment (4)
WARNINGS AND PRECAUTIONS
- Myocardial ischemia/infarction and Prinzmetal’s angina: Perform cardiac evaluation in patients with multiple cardiovascular risk factors (5.1)
- Arrhythmias: Discontinue IMITREX if occurs (5.2)
- Chest/throat/neck/jaw pain, tightness, pressure, or heaviness: Generally not associated with myocardial ischemia; evaluate for coronary artery disease in patients at high risk (5.3)
- Cerebral hemorrhage, subarachnoid hemorrhage, and stroke: Discontinue IMITREX if occurs (5.4)
- Gastrointestinal ischemic reactions and peripheral vasospastic reactions: Discontinue IMITREX if occurs (5.5)
- Medication overuse headache: Detoxification may be necessary (5.6)
- Serotonin syndrome: Discontinue IMITREX if occurs (5.7)
- Seizures: Use with caution in patients with epilepsy or a lowered seizure threshold (5.10)
ADVERSE REACTIONS
Most common adverse reactions (≥5% and >placebo) were injection site reactions, tingling, dizziness/vertigo, warm/hot sensation, burning sensation, feeling of heaviness, pressure sensation, flushing, feeling of tightness, and numbness (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
Pregnancy: Based on animal data, may cause fetal harm (8.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Administration Using the IMITREX STATdose Pen
2.3 Administration of Doses of IMITREX Other than 4 or 6 mg
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina
5.2 Arrhythmias
5.3 Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure
5.4 Cerebrovascular Events
5.5 Other Vasospasm Reactions
5.6 Medication Overuse Headache
5.7 Serotonin Syndrome
5.8 Increase in Blood Pressure
5.9 Hypersensitivity Reactions
5.10 Seizures
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Ergot-Containing Drugs
7.2 Monoamine Oxidase-A Inhibitors
7.3 Other 5-HT1 Agonists
7.4 Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Migraine
14.2 Cluster Headache
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
IMITREX injection is indicated in adults for (1) the acute treatment of migraine, with or without aura, and (2) the acute treatment of cluster headache.
Limitations of Use:
- Use only if a clear diagnosis of migraine or cluster headache has been established. If a patient has no response to the first migraine or cluster headache attack treated with IMITREX injection, reconsider the diagnosis before IMITREX injection is administered to treat any subsequent attacks.
- IMITREX injection is not indicated for the prevention of migraine or cluster headache attacks.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
The maximum single recommended adult dose of IMITREX injection for the acute treatment of migraine or cluster headache is 6 mg injected subcutaneously. For the treatment of migraine, if side effects are dose limiting, lower doses (1 mg to 5 mg) may be used [see Clinical Studies (14.1)]. For the treatment of cluster headache, the efficacy of lower doses has not been established.
The maximum cumulative dose that may be given in 24 hours is 12 mg, two 6-mg injections separated by at least 1 hour. A second 6-mg dose should only be considered if some response to a first injection was observed.
2.2 Administration Using the IMITREX STATdose Pen
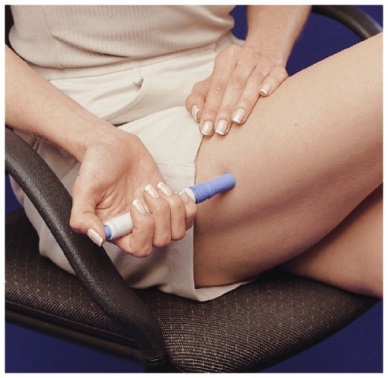
An autoinjector device (IMITREX STATdose Pen) is available for use with 4-mg and 6-mg prefilled syringe cartridges. With this device, the needle penetrates approximately 1/4 inch (5 to 6 mm). The injection is intended to be given subcutaneously, and intramuscular or intravascular delivery must be avoided. Instruct patients on the proper use of IMITREX STATdose Pen and direct them to use injection sites with an adequate skin and subcutaneous thickness to accommodate the length of the needle.
The needle shield of the prefilled syringe contains dry natural rubber (a latex derivative) [see Warnings and Precautions(5.9)].
2.3 Administration of Doses of IMITREX Other than 4 or 6 mg
In patients receiving doses other than 4 mg or 6 mg, use the 6-mg single-dose vial; do not use the IMITREX STATdose Pen. Visually inspect the vial for particulate matter and discoloration before administration. Do not use if particulates and discolorations are noted.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
IMITREX injection is contraindicated in patients with:
- Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary artery vasospasm, including Prinzmetal’s angina [see Warnings and Precautions (5.1)].
- Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders [see Warnings and Precautions (5.2)].
- History of stroke or transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at a higher risk of stroke [see Warnings and Precautions (5.4)].
- Peripheral vascular disease [see Warnings and Precautions (5.5)].
- Ischemic bowel disease [see Warnings and Precautions (5.5)].
- Uncontrolled hypertension [see Warnings and Precautions (5.8)].
- Recent use (i.e., within 24 hours) of ergotamine-containing medication, ergot-type medication (such as dihydroergotamine or methysergide), or another 5-hydroxytryptamine1 (5-HT1) agonist [see Drug Interactions (7.1, 7.3)].
- Concurrent administration of a monoamine oxidase (MAO)-A inhibitor or recent (within 2 weeks) use of an MAO-A inhibitor [see Drug Interactions (7.2), Clinical Pharmacology (12.3)].
- Hypersensitivity to IMITREX (angioedema and anaphylaxis seen) [see Warnings and Precautions (5.9)].
- Severe hepatic impairment [see Clinical Pharmacology (12.3)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina
The use of IMITREX injection is contraindicated in patients with ischemic or vasospastic CAD. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of IMITREX injection. Some of these reactions occurred in patients without known CAD. IMITREX injection may cause coronary artery vasospasm (Prinzmetal’s angina), even in patients without a history of CAD.
Perform a cardiovascular evaluation in triptan-naive patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving IMITREX injection. If there is evidence of CAD or coronary artery vasospasm, IMITREX injection is contraindicated. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administering the first dose of IMITREX injection in a medically supervised setting and performing an electrocardiogram (ECG) immediately following administration of IMITREX injection. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of IMITREX injection.
5.2 Arrhythmias
Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue IMITREX injection if these disturbances occur. IMITREX injection is contraindicated in patients with Wolff-Parkinson-White syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders.
5.3 Chest, Throat, Neck, and/or Jaw Pain/Tightness/Pressure
Sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with IMITREX injection and are usually non-cardiac in origin. However, perform a cardiac evaluation if these patients are at high cardiac risk. The use of IMITREX injection is contraindicated in patients with CAD and those with Prinzmetal’s variant angina.
5.4 Cerebrovascular Events
Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, TIA). Discontinue IMITREX injection if a cerebrovascular event occurs.
Before treating headaches in patients not previously diagnosed with migraine or cluster headache or in patients who present with atypical symptoms, exclude other potentially serious neurological conditions. IMITREX injection is contraindicated in patients with a history of stroke or TIA.
5.5 Other Vasospasm Reactions
IMITREX injection may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1 agonist, rule out a vasospastic reaction before receiving additional injections of IMITREX.
Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists has not been clearly established.
5.6 Medication Overuse Headache
Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or combination of these drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
5.7 Serotonin Syndrome
Serotonin syndrome may occur with IMITREX injection, particularly during coadministration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.4)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. Discontinue IMITREX injection if serotonin syndrome is suspected.
5.8 Increase in Blood Pressure
Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients treated with 5-HT1 agonists, including patients without a history of hypertension. Monitor blood pressure in patients treated with IMITREX. IMITREX injection is contraindicated in patients with uncontrolled hypertension.
5.9 Hypersensitivity Reactions
Hypersensitivity reactions, including angioedema and anaphylaxis, have occurred in patients receiving IMITREX. Such reactions can be life-threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens. IMITREX injection is contraindicated in patients with a history of hypersensitivity reaction to IMITREX.
The needle shield of the prefilled syringe contains dry natural rubber (a latex derivative) that has the potential to cause allergic reactions in latex-sensitive individuals.
5.10 Seizures
Seizures have been reported following administration of IMITREX. Some have occurred in patients with either a history of seizures or concurrent conditions predisposing to seizures. There are also reports in patients where no such predisposing factors are apparent. IMITREX injection should be used with caution in patients with a history of epilepsy or conditions associated with a lowered seizure threshold.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina [see Warnings and Precautions (5.1)]
- Arrhythmias [see Warnings and Precautions (5.2)]
- Chest, throat, neck, and/or jaw pain/tightness/pressure [see Warnings and Precautions (5.3)]
- Cerebrovascular events [see Warnings and Precautions (5.4)]
- Other vasospasm reactions [see Warnings and Precautions (5.5)]
- Medication overuse headache [see Warnings and Precautions (5.6)]
- Serotonin syndrome [see Warnings and Precautions (5.7)]
- Increase in blood pressure [see Warnings and Precautions (5.8)]
- Hypersensitivity reactions [see Contraindications (4), Warnings and Precautions (5.9)]
- Seizures [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Migraine Headache
Table 1 lists adverse reactions that occurred in 2 U.S. placebo‑controlled clinical trials in patients with migraines (Studies 2 and 3) following either a single 6‑mg dose of IMITREX injection or placebo. Only reactions that occurred at a frequency of 2% or more in groups treated with IMITREX injection 6 mg and that occurred at a frequency greater than the placebo group are included in Table 1.
Table 1. Adverse Reactions in Pooled Placebo-Controlled Trials in Patients with Migraine (Studies 2 and 3) Adverse Reaction
IMITREX Injection
6 mg Subcutaneous
(n = 547)
%
Placebo
(n = 370)
%
Atypical sensations
42
9
- Tingling
14
3
- Warm/hot sensation
11
4
- Burning sensation
7
<1
- Feeling of heaviness
7
1
- Pressure sensation
7
2
- Feeling of tightness
5
<1
- Numbness
5
2
- Feeling strange
2
<1
- Tight feeling in head
2
<1
Cardiovascular
- Flushing
7
2
- Chest discomfort
5
1
- Tightness in chest
3
<1
- Pressure in chest
2
<1
Ear, nose, and throat
- Throat discomfort
3
<1
- Discomfort: nasal cavity/sinuses
2
<1
Injection site reactiona
59
24
Miscellaneous
- Jaw discomfort
2
0
Musculoskeletal
- Weakness
5
<1
- Neck pain/stiffness
5
<1
- Myalgia
2
<1
Neurological
- Dizziness/vertigo
12
4
- Drowsiness/sedation
3
2
- Headache
2
<1
Skin
- Sweating
2
1
- a Includes injection site pain, stinging/burning, swelling, erythema, bruising, bleeding.
The incidence of adverse reactions in controlled clinical trials was not affected by gender or age of the patients. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Cluster Headache
In the controlled clinical trials assessing the efficacy of IMITREX injection as a treatment for cluster headache (Studies 4 and 5), no new significant adverse reactions were detected that had not already been identified in trials of IMITREX in patients with migraine.
Overall, the frequency of adverse reactions reported in the trials of cluster headache was generally lower than in the migraine trials. Exceptions include reports of paresthesia (5% IMITREX injection, 0% placebo), nausea and vomiting (4% IMITREX injection, 0% placebo), and bronchospasm (1% IMITREX injection, 0% placebo).
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of IMITREX tablets, IMITREX nasal spray, and IMITREX injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular
Hypotension, palpitations.
Neurological
Dystonia, tremor.
-
7 DRUG INTERACTIONS
7.1 Ergot-Containing Drugs
Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ergot-type medications (like dihydroergotamine or methysergide) and IMITREX injection within 24 hours of each other is contraindicated.
7.2 Monoamine Oxidase-A Inhibitors
MAO-A inhibitors increase systemic exposure by 2-fold. Therefore, the use of IMITREX injection in patients receiving MAO-A inhibitors is contraindicated [see Clinical Pharmacology (12.3)].
7.3 Other 5-HT1 Agonists
Because their vasospastic effects may be additive, coadministration of IMITREX injection and other 5-HT1 agonists (e.g., triptans) within 24 hours of each other is contraindicated.
7.4 Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome
Cases of serotonin syndrome have been reported during coadministration of triptans and SSRIs, SNRIs, TCAs, and MAO inhibitors [see Warnings and Precautions (5.7)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Data from a prospective pregnancy exposure registry and epidemiological studies of pregnant women have not detected an increased frequency of birth defects or a consistent pattern of birth defects among women exposed to sumatriptan compared with the general population (see Data). In developmental toxicity studies in rats and rabbits, oral administration of sumatriptan to pregnant animals was associated with embryolethality, fetal abnormalities, and pup mortality. When administered by the intravenous route to pregnant rabbits, sumatriptan was embryolethal (see Data).
In the U.S. general population, the estimated background risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The reported rate of major birth defects among deliveries to women with migraine ranged from 2.2% to 2.9% and the reported rate of miscarriage was 17%, which were similar to rates reported in women without migraine.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk: Several studies have suggested that women with migraine may be at increased risk of preeclampsia during pregnancy.
Data
Human Data: The Sumatriptan/Naratriptan/Treximet (sumatriptan and naproxen sodium) Pregnancy Registry, a population-based international prospective study, collected data for sumatriptan from January 1996 to September 2012. The Registry documented outcomes of 626 infants and fetuses exposed to sumatriptan during pregnancy (528 with earliest exposure during the first trimester, 78 during the second trimester, 16 during the third trimester, and 4 unknown). The occurrence of major birth defects (excluding fetal deaths and induced abortions without reported defects and all spontaneous pregnancy losses) during first-trimester exposure to sumatriptan was 4.2% (20/478 [95% CI: 2.6% to 6.5%]) and during any trimester of exposure was 4.2% (24/576 [95% CI: 2.7% to 6.2%]). The sample size in this study had 80% power to detect at least a 1.73- to 1.91-fold increase in the rate of major malformations. The number of exposed pregnancy outcomes accumulated during the registry was insufficient to support definitive conclusions about overall malformation risk or for making comparisons of the frequencies of specific birth defects. Of the 20 infants with reported birth defects after exposure to sumatriptan in the first trimester, 4 infants had ventricular septal defects, including one infant who was exposed to both sumatriptan and naratriptan, and 3 infants had pyloric stenosis. No other birth defect was reported for more than 2 infants in this group.
In a study using data from the Swedish Medical Birth Register, live births to women who reported using triptans or ergots during pregnancy were compared with those of women who did not. Of the 2,257 births with first-trimester exposure to sumatriptan, 107 infants were born with malformations (relative risk 0.99 [95% CI: 0.91 to 1.21]). A study using linked data from the Medical Birth Registry of Norway to the Norwegian Prescription Database compared pregnancy outcomes in women who redeemed prescriptions for triptans during pregnancy, as well as a migraine disease comparison group who redeemed prescriptions for sumatriptan before pregnancy only, compared with a population control group. Of the 415 women who redeemed prescriptions for sumatriptan during the first trimester, 15 had infants with major congenital malformations (OR 1.16 [95% CI: 0.69 to 1.94]) while for the 364 women who redeemed prescriptions for sumatriptan before, but not during, pregnancy, 20 had infants with major congenital malformations (OR 1.83 [95% CI: 1.17 to 2.88]), each compared with the population comparison group. Additional smaller observational studies evaluating use of sumatriptan during pregnancy have not suggested an increased risk of teratogenicity.
Animal Data: Oral administration of sumatriptan to pregnant rats during the period of organogenesis resulted in an increased incidence of fetal blood vessel (cervicothoracic and umbilical) abnormalities. The highest no-effect dose for embryofetal developmental toxicity in rats was 60 mg/kg/day. Oral administration of sumatriptan to pregnant rabbits during the period of organogenesis resulted in increased incidences of embryolethality and fetal cervicothoracic vascular and skeletal abnormalities. Intravenous administration of sumatriptan to pregnant rabbits during the period of organogenesis resulted in an increased incidence of embryolethality. The highest oral and intravenous no-effect doses for developmental toxicity in rabbits were 15 and 0.75 mg/kg/day, respectively.
Oral administration of sumatriptan to rats prior to and throughout gestation resulted in embryofetal toxicity (decreased body weight, decreased ossification, increased incidence of skeletal abnormalities). The highest no-effect dose was 50 mg/kg/day. In offspring of pregnant rats treated orally with sumatriptan during organogenesis, there was a decrease in pup survival. The highest no-effect dose for this effect was 60 mg/kg/day. Oral treatment of pregnant rats with sumatriptan during the latter part of gestation and throughout lactation resulted in a decrease in pup survival. The highest no-effect dose for this finding was 100 mg/kg/day.
8.2 Lactation
Risk Summary
Sumatriptan is excreted in human milk following subcutaneous administration (see Data). There are no data on the effects of sumatriptan on the breastfed infant or the effects of sumatriptan on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for IMITREX injection and any potential adverse effects on the breastfed infant from sumatriptan or from the underlying maternal condition.
Clinical Considerations
Infant exposure to sumatriptan can be minimized by avoiding breastfeeding for 12 hours after treatment with IMITREX injection.
Data
Following subcutaneous administration of a 6-mg dose of IMITREX injection in 5 lactating volunteers, sumatriptan was present in milk.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. IMITREX injection is not recommended for use in patients younger than 18 years of age.
Two controlled clinical trials evaluated IMITREX nasal spray (5 to 20 mg) in 1,248 pediatric migraineurs aged 12 to 17 years who treated a single attack. The trials did not establish the efficacy of IMITREX nasal spray compared with placebo in the treatment of migraine in pediatric patients. Adverse reactions observed in these clinical trials were similar in nature to those reported in clinical trials in adults.
Five controlled clinical trials (2 single-attack trials, 3 multiple-attack trials) evaluating oral IMITREX (25 to 100 mg) in pediatric patients aged 12 to 17 years enrolled a total of 701 pediatric migraineurs. These trials did not establish the efficacy of oral IMITREX compared with placebo in the treatment of migraine in pediatric patients. Adverse reactions observed in these clinical trials were similar in nature to those reported in clinical trials in adults. The frequency of all adverse reactions in these patients appeared to be both dose- and age‑dependent, with younger patients reporting reactions more commonly than older pediatric patients.
Postmarketing experience documents that serious adverse reactions have occurred in the pediatric population after use of subcutaneous, oral, and/or intranasal IMITREX. These reports include reactions similar in nature to those reported rarely in adults, including stroke, visual loss, and death. A myocardial infarction has been reported in a 14-year-old male following the use of oral IMITREX; clinical signs occurred within 1 day of drug administration. Clinical data to determine the frequency of serious adverse reactions in pediatric patients who might receive subcutaneous, oral, or intranasal IMITREX are not presently available.
8.5 Geriatric Use
Clinical trials of IMITREX injection did not include sufficient numbers of patients aged 65 years and older to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
A cardiovascular evaluation is recommended for geriatric patients who have other cardiovascular risk factors (e.g., diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving IMITREX injection [see Warnings and Precautions (5.1)].
-
10 OVERDOSAGE
Coronary vasospasm was observed after intravenous administration of IMITREX injection [see Contraindications (4)]. Overdoses would be expected from animal data (dogs at 0.1 g/kg, rats at 2 g/kg) to possibly cause convulsions, tremor, inactivity, erythema of the extremities, reduced respiratory rate, cyanosis, ataxia, mydriasis, injection site reactions (desquamation, hair loss, and scab formation), and paralysis.
The elimination half-life of sumatriptan is about 2 hours [see Clinical Pharmacology (12.3)]; therefore, monitoring of patients after overdose with IMITREX injection should continue for at least 10 hours or while symptoms or signs persist.
It is unknown what effect hemodialysis or peritoneal dialysis has on the serum concentrations of sumatriptan.
-
11 DESCRIPTION
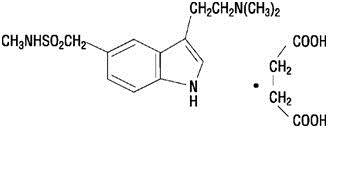
IMITREX injection contains sumatriptan succinate, a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate is chemically designated as 3-[2-(dimethylamino)ethyl]-N-methyl-indole-5-methanesulfonamide succinate (1:1), and it has the following structure:
The empirical formula is C14H21N3O2SC4H6O4, representing a molecular weight of 413.5. Sumatriptan succinate is a white to off-white powder that is readily soluble in water and in saline.
IMITREX injection is a clear, colorless to pale yellow, sterile, nonpyrogenic solution for subcutaneous injection. Each 0.5 mL of IMITREX injection 8-mg/mL solution contains 4 mg of sumatriptan (base) as the succinate salt and 3.8 mg of sodium chloride, USP in Water for Injection, USP. Each 0.5 mL of IMITREX injection 12-mg/mL solution contains 6 mg of sumatriptan (base) as the succinate salt and 3.5 mg of sodium chloride, USP in Water for Injection, USP. The pH range of both solutions is approximately 4.2 to 5.3. The osmolality of both injections is 291 mOsmol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine and cluster headaches through agonist effects at the 5-HT1B/1D receptors on intracranial blood vessels and sensory nerves of the trigeminal system, which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
12.2 Pharmacodynamics
Blood Pressure
Significant elevation in blood pressure, including hypertensive crisis, has been reported in patients with and without a history of hypertension [see Warnings and Precautions (5.8)].
Peripheral (Small) Arteries
In healthy volunteers (N = 18), a trial evaluating the effects of sumatriptan on peripheral (small vessel) arterial reactivity failed to detect a clinically significant increase in peripheral resistance.
Heart Rate
Transient increases in blood pressure observed in some patients in clinical trials carried out during sumatriptan’s development as a treatment for migraine were not accompanied by any clinically significant changes in heart rate.
12.3 Pharmacokinetics
Absorption
The bioavailability of sumatriptan via subcutaneous site injection to 18 healthy male subjects was 97% ± 16% of that obtained following intravenous injection.
After a single 6-mg subcutaneous manual injection into the deltoid area of the arm in 18 healthy males (age: 24 ± 6 years, weight: 70 kg), the maximum serum concentration (Cmax) of sumatriptan was (mean ± standard deviation) 74 ± 15 ng/mL and the time to peak concentration (Tmax) was 12 minutes after injection (range: 5 to 20 minutes). In this trial, the same dose injected subcutaneously in the thigh gave a Cmax of 61 ± 15 ng/mL by manual injection versus 52 ± 15 ng/mL by autoinjector techniques. The Tmax or amount absorbed was not significantly altered by either the site or technique of injection.
Distribution
Protein binding, determined by equilibrium dialysis over the concentration range of 10 to 1,000 ng/mL is low, approximately 14% to 21%. The effect of sumatriptan on the protein binding of other drugs has not been evaluated.
Following a 6-mg subcutaneous injection into the deltoid area of the arm in 9 males (mean age: 33 years, mean weight: 77 kg) the volume of distribution central compartment of sumatriptan was 50 ± 8 liters and the distribution half‑life was 15 ± 2 minutes.
Metabolism
In vitro studies with human microsomes suggest that sumatriptan is metabolized by MAO, predominantly the A isoenzyme. Most of a radiolabeled dose of sumatriptan excreted in the urine is the major metabolite indole acetic acid (IAA) or the IAA glucuronide, both of which are inactive.
Elimination
After a single 6-mg subcutaneous dose, 22% ± 4% was excreted in the urine as unchanged sumatriptan and 38% ± 7% as the IAA metabolite.
Following a 6-mg subcutaneous injection into the deltoid area of the arm, the systemic clearance of sumatriptan was 1,194 ± 149 mL/min and the terminal half-life was 115 ± 19 minutes.
Specific Populations
Age: The pharmacokinetics of sumatriptan in the elderly (mean age: 72 years, 2 males and 4 females) and in subjects with migraine (mean age: 38 years, 25 males and 155 females) were similar to that in healthy male subjects (mean age: 30 years).
Patients with Hepatic Impairment: The effect of mild to moderate hepatic disease on the pharmacokinetics of subcutaneously administered sumatriptan has been evaluated. There were no significant differences in the pharmacokinetics of subcutaneously administered sumatriptan in moderately hepatically impaired subjects compared with healthy controls. The pharmacokinetics of subcutaneously administered sumatriptan in patients with severe hepatic impairment has not been studied. The use of IMITREX injection in this population is contraindicated [see Contraindications (4)].
Racial Groups: The systemic clearance and Cmax of subcutaneous sumatriptan were similar in black (n = 34) and Caucasian (n = 38) healthy male subjects.
Drug Interaction Studies
Monoamine Oxidase-A Inhibitors: In a trial of 14 healthy females, pretreatment with an MAO-A inhibitor decreased the clearance of subcutaneous sumatriptan, resulting in a 2-fold increase in the area under the sumatriptan plasma concentration-time curve (AUC), corresponding to a 40% increase in elimination half-life.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In carcinogenicity studies in mouse and rat, sumatriptan was administered orally for 78 weeks and 104 weeks, respectively, at doses up to 160 mg/kg/day (the high dose in rat was reduced from 360 mg/kg/day during Week 21). The highest dose to mice and rats was approximately 130 and 260 times the single MRHD of 6 mg administered subcutaneously on a mg/m2 basis. There was no evidence in either species of an increase in tumors related to sumatriptan administration.
Mutagenesis
Sumatriptan was negative in in vitro (bacterial reverse mutation [Ames], gene cell mutation in Chinese hamster V79/HGPRT, chromosomal aberration in human lymphocytes) and in vivo (rat micronucleus) assays.
Impairment of Fertility
When sumatriptan (5, 50, 500 mg/kg/day) was administered orally to male and female rats prior to and throughout the mating period, there was a treatment-related decrease in fertility secondary to a decrease in mating in animals treated with doses greater than 5 mg/kg/day. It is not clear whether this finding was due to an effect on males or females or both.
When sumatriptan was administered by subcutaneous injection to male and female rats prior to and throughout the mating period, there was no evidence of impaired fertility at doses up to 60 mg/kg/day.
13.2 Animal Toxicology and/or Pharmacology
Corneal Opacities
Dogs receiving oral sumatriptan developed corneal opacities and defects in the corneal epithelium. Corneal opacities were seen at the lowest dose tested, 2 mg/kg/day, and were present after 1 month of treatment. Defects in the corneal epithelium were noted in a 60-week study. Earlier examinations for these toxicities were not conducted and no-effect doses were not established; however, the relative plasma exposure at the lowest dose tested was approximately 3 times the human exposure after a 6-mg subcutaneous dose.
-
14 CLINICAL STUDIES
14.1 Migraine
In controlled clinical trials enrolling more than 1,000 patients during migraine attacks who were experiencing moderate or severe pain and 1 or more of the symptoms enumerated in Table 3, onset of relief began as early as 10 minutes following a 6-mg IMITREX injection. Lower doses of IMITREX injection may also prove effective, although the proportion of patients obtaining adequate relief was decreased and the latency to that relief is greater with lower doses.
In Study 1, 6 different doses of IMITREX injection (n = 30 each group) were compared with placebo (n = 62) in a single-attack, parallel-group design; the dose-response relationship was found to be as shown in Table 2.
Table 2. Proportion of Patients with Migraine Relief and Incidence of Adverse Reactions by Time and by Dose of IMITREX in Study 1 Dose of IMITREX Injection
Percent Patients with Reliefa
Adverse Reactions Incidence (%)
at 10 Minutes
at 30 Minutes
at 1 Hour
at 2 Hours
Placebo
5
15
24
21
55
1 mg
10
40
43
40
63
2 mg
7
23
57
43
63
3 mg
17
47
57
60
77
4 mg
13
37
50
57
80
6 mg
10
63
73
70
83
8 mg
23
57
80
83
93
- a Relief is defined as the reduction of moderate or severe pain to no or mild pain after dosing without use of rescue medication.
In 2 randomized, placebo-controlled clinical trials of IMITREX injection 6 mg in 1,104 patients with moderate or severe migraine pain (Studies 2 and 3), the onset of relief was less than 10 minutes. Headache relief, as defined by a reduction in pain from severe or moderately severe to mild or no headache, was achieved in 70% of the patients within 1 hour of a single 6-mg subcutaneous dose of IMITREX injection. Approximately 82% and 65% of patients treated with IMITREX 6 mg had headache relief and were pain free within 2 hours, respectively.
Table 3 shows the 1- and 2-hour efficacy results for IMITREX injection 6 mg in Studies 2 and 3.
Table 3. Proportion of Patients with Pain Relief and Relief of Migraine Symptoms after 1 and 2 Hours of Treatment in Studies 2 and 3 1-Hour Data
Study 2
Study 3
Placebo
(n = 190)
IMITREX
6 mg
(n = 384)
Placebo
(n = 180)
IMITREX 6 mg
(n = 350)
Patients with pain relief (Grade 0/1)
18%
70%a
26%
70%a
Patients with no pain
5%
48%a
13%
49%a
Patients without nausea
48%
73%a
50%
73%a
Patients without photophobia
23%
56%a
25%
58%a
Patients with little or no clinical disabilityb
34%
76%a
34%
76%a
2-Hour Data
Study 2
Study 3
Placeboc
IMITREX
6 mgd
Placeboc
IMITREX
6 mgd
Patients with pain relief (Grade 0/1)
31%
81%a
39%
82%a
Patients with no pain
11%
63%a
19%
65%a
Patients without nausea
56%
82%a
63%
81%a
Patients without photophobia
31%
72%a
35%
71%a
Patients with little or no clinical disabilityb
42%
85%a
49%
84%a
- a P<0.05 versus placebo.
- b A successful outcome in terms of clinical disability was defined prospectively as ability to work mildly impaired or ability to work and function normally.
- c Includes patients that may have received an additional placebo injection 1 hour after the initial injection.
- d Includes patients that may have received an additional 6 mg of IMITREX injection 1 hour after the initial injection.
IMITREX injection also relieved photophobia, phonophobia (sound sensitivity), nausea, and vomiting associated with migraine attacks. Similar efficacy was seen when patients self-administered IMITREX injection using the IMITREX STATdose Pen.
The efficacy of IMITREX injection was unaffected by whether or not the migraine was associated with aura, duration of attack, gender or age of the patient, or concomitant use of common migraine prophylactic drugs (e.g., beta-blockers).
14.2 Cluster Headache
The efficacy of IMITREX injection in the acute treatment of cluster headache was demonstrated in 2 randomized, double-blind, placebo-controlled, 2-period crossover trials (Studies 4 and 5). Patients aged 21 to 65 years were enrolled and were instructed to treat a moderate to very severe headache within 10 minutes of onset. Headache relief was defined as a reduction in headache severity to mild or no pain. In both trials, the proportion of individuals gaining relief at 10 or 15 minutes was significantly greater among patients receiving 6 mg of IMITREX injection compared with those who received placebo (see Table 4).
Table 4. Proportion of Patients with Cluster Headache Relief by Time in Studies 4 and 5 Study 4
Study 5
Placebo
(n = 39)
IMITREX
6 mg
(n = 39)
Placebo
(n = 88)
IMITREX
6 mg
(n = 92)
Patients with pain relief (no/mild)
- 5 Minutes post-injection
8%
21%
7%
23%a
- 10 Minutes post-injection
10%
49%a
25%
49%a
- 15 Minutes post-injection
26%
74%a
35%
75%a
- a P<0.05.
- n = Number of headaches treated.
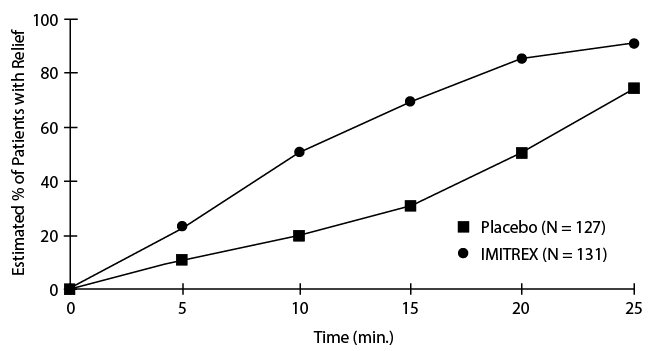
An estimate of the cumulative probability of a patient with a cluster headache obtaining relief after being treated with either IMITREX injection or placebo is presented in Figure 1.
Figure 1. Time to Relief of Cluster Headache from Time of Injectiona
- a The figure uses Kaplan-Meier (product limit) Survivorship Plot. Patients taking rescue medication were censored at 15 minutes.
The plot was constructed with data from patients who either experienced relief or did not require (request) rescue medication within a period of 2 hours following treatment. As a consequence, the data in the plot are derived from only a subset of the 258 headaches treated (rescue medication was required in 52 of the 127 placebo-treated headaches and 18 of the 131 headaches treated with IMITREX injection).
Other data suggest that treatment with IMITREX injection is not associated with an increase in early recurrence of headache and has little effect on the incidence of later-occurring headaches (i.e., those occurring after 2, but before 18 or 24 hours).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
IMITREX injection contains sumatriptan (base) as the succinate salt and is supplied as a clear, colorless to pale yellow, sterile, nonpyrogenic solution as follows:
Prefilled Syringe and/or Autoinjector Pen:
The needle shield of the prefilled syringe contains dry natural rubber (a latex derivative) that has the potential to cause allergic reactions in latex-sensitive individuals.
Each pack contains a Patient Information and Instructions for Use leaflet.
- IMITREX STATdose System, 4 mg, containing 1 IMITREX STATdose Pen, 2 prefilled single-dose syringe cartridges, and 1 carrying case (NDC: 0173-0739-00).
- IMITREX STATdose System, 6 mg, containing 1 IMITREX STATdose Pen, 2 prefilled single-dose syringe cartridges, and 1 carrying case (NDC: 0173-0479-00).
- Two 4-mg single-dose prefilled syringe cartridges for use with IMITREX STATdose System (NDC: 0173-0739-02).
- Two 6-mg single-dose prefilled syringe cartridges for use with IMITREX STATdose System (NDC: 0173-0478-00).
Single-Dose Vial:
- IMITREX injection single-dose vial (6 mg/0.5 mL) in cartons containing 5 vials (NDC: 0173-0449-02).
Store between 2° and 30°C (36° and 86°F). Protect from light.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Risk of Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other Vasospasm-Related Events, Arrhythmias, and Cerebrovascular Events
Inform patients that IMITREX injection may cause serious cardiovascular side effects such as myocardial infarction or stroke. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, irregular heartbeat, significant rise in blood pressure, weakness, and slurring of speech, and should ask for medical advice if any indicative sign or symptoms are observed. Apprise patients of the importance of this follow-up [see Warnings and Precautions (5.1, 5.2, 5.4, 5.5, 5.8)].
Hypersensitivity Reactions
Inform patients that anaphylactic reactions have occurred in patients receiving IMITREX injection. Such reactions can be life-threatening or fatal. In general, anaphylactic reactions to drugs are more likely to occur in individuals with a history of sensitivity to multiple allergens [see Contraindications (4), Warnings and Precautions (5.9)]. Inform latex-sensitive patients that the needle shield of the IMITREX prefilled syringe contains dry natural rubber (a derivative of latex) that may cause allergic reactions in individuals sensitive to latex.
Concomitant Use with Other Triptans or Ergot Medications
Inform patients that use of IMITREX injection within 24 hours of another triptan or an ergot-type medication (including dihydroergotamine or methysergide) is contraindicated [see Contraindications (4), Drug Interactions (7.1, 7.3)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with the use of IMITREX injection or other triptans, particularly during combined use with SSRIs, SNRIs, TCAs, and MAO inhibitors [see Warnings and Precautions (5.7), Drug Interactions (7.4)].
Medication Overuse Headache
Inform patients that use of acute migraine drugs for 10 or more days per month may lead to an exacerbation of headache and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary) [see Warnings and Precautions (5.6)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant [see Use in Specific Populations (8.1)].
Lactation
Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed [see Use in Specific Populations (8.2)].
Ability to Perform Complex Tasks
Treatment with IMITREX injection may cause somnolence and dizziness; instruct patients to evaluate their ability to perform complex tasks after administration of IMITREX injection.
How to Use IMITREX injection
Instruct patients to read the Instructions for Use before starting therapy. Provide patients instruction on the proper use of IMITREX injection if they are able to self-administer IMITREX injection in medically unsupervised situations. Instruct patients on storage and disposal of the pen [see How Supplied/Storage and Handling (16)].
Inform patients that the needle in the IMITREX STATdose Pen penetrates approximately 1/4 of an inch (5 to 6 mm). Inform patients that the injection is intended to be given subcutaneously and intramuscular or intravascular delivery should be avoided. Instruct patients to use injection sites with an adequate skin and subcutaneous thickness to accommodate the length of the needle.
Trademarks are owned by or licensed to the GSK group of companies.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2018 GSK group of companies or its licensor.
IMJ:8PI
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
IMITREX (IM-i-trex)
(sumatriptan succinate)
injection
What is the most important information I should know about IMITREX?
IMITREX can cause serious side effects, including:
Heart attack and other heart problems. Heart problems may lead to death.
Stop taking IMITREX and get emergency medical help right away if you have any of the following symptoms of a heart attack:
- discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back
- severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw
- pain or discomfort in your arms, back, neck, jaw, or stomach
- shortness of breath with or without chest discomfort
- breaking out in a cold sweat
- nausea or vomiting
- feeling lightheaded
IMITREX is not for people with risk factors for heart disease unless a heart exam is done and shows no problem. You have a higher risk for heart disease if you:
- have high blood pressure
- have high cholesterol levels
- smoke
- are overweight
- have diabetes
- have a family history of heart disease
What is IMITREX?
IMITREX injection is a prescription medicine used to treat acute migraine headaches with or without aura and acute cluster headaches in adults who have been diagnosed with migraine or cluster headaches.
IMITREX is not used to treat other types of headaches such as hemiplegic (that make you unable to move on one side of your body) or basilar (rare form of migraine with aura) migraines.
IMITREX is not used to prevent or decrease the number of migraine or cluster headaches you have.
It is not known if IMITREX is safe and effective in children under 18 years of age.
Do not take IMITREX if you have:
- heart problems or a history of heart problems.
- narrowing of blood vessels to your legs, arms, stomach, or kidneys (peripheral vascular disease).
- uncontrolled high blood pressure.
- severe liver problems.
- hemiplegic migraines or basilar migraines. If you are not sure if you have these types of migraines, ask your healthcare provider.
- had a stroke, transient ischemic attacks (TIAs), or problems with your blood circulation.
- taken any of the following medicines in the last 24 hours:
- o almotriptan (AXERT)
- o frovatriptan (FROVA)
- o rizatriptan (MAXALT, MAXALT-MLT)
- o ergotamines (CAFERGOT, ERGOMAR, MIGERGOT)
- o eletriptan (RELPAX)
- o naratriptan (AMERGE)
- o sumatriptan and naproxen (TREXIMET)
- o dihydroergotamine (D.H.E. 45, MIGRANAL)
- Ask your healthcare provider if you are not sure if your medicine is listed above.
- an allergy to sumatriptan or any of the ingredients in IMITREX. See the end of this leaflet for a complete list of ingredients in IMITREX.
Before taking IMITREX, tell your healthcare provider about all of your medical conditions, including if you:
- have high blood pressure
- have high cholesterol
- have diabetes
- smoke
- are overweight
- have heart problems or family history of heart problems or stroke
- have kidney problems
- have liver problems
- are allergic to latex
- have had epilepsy or seizures
- are not using effective birth control
- are pregnant or plan to become pregnant. It is not known if IMITREX can harm your unborn baby.
- are breastfeeding or plan to breastfeed. IMITREX passes into your breast milk. It is not known if this can harm your baby. Talk with your healthcare provider about the best way to feed your baby if you take IMITREX.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
IMITREX and certain other medicines can affect each other, causing serious side effects.
Especially tell your healthcare provider if you take antidepressant medicines called:
- selective serotonin reuptake inhibitors (SSRIs)
- serotonin norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants (TCAs)
- monoamine oxidase inhibitors (MAOIs)
Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I take IMITREX?
- Certain people should take their first dose of IMITREX in their healthcare provider’s office or in another medical setting. Ask your healthcare provider if you should take your first dose in a medical setting.
- Use IMITREX exactly as your healthcare provider tells you to use it.
- Your healthcare provider may change your dose. Do not change your dose without first talking with your healthcare provider.
- For adults, the usual dose is a single injection given just below the skin.
- You should give an injection as soon as the symptoms of your headache start, but it may be given at any time during a migraine or cluster headache attack.
- If you did not get any relief after the first injection, do not give a second injection without first talking with your healthcare provider.
- If your headache comes back or you only get some relief after your first injection, you can take a second injection 1 hour after the first injection, but not sooner.
- Do not take more than 12 mg in a 24‑hour period.
- If you use too much IMITREX, call your healthcare provider or go to the nearest hospital emergency room right away.
- You should write down when you have headaches and when you take IMITREX so you can talk with your healthcare provider about how IMITREX is working for you.
What should I avoid while taking IMITREX?
IMITREX can cause dizziness, weakness, or drowsiness. If you have these symptoms, do not drive a car, use machinery, or do anything where you need to be alert.
What are the possible side effects of IMITREX?
IMITREX may cause serious side effects. See “What is the most important information I should know about IMITREX?”
These serious side effects include:
- changes in color or sensation in your fingers and toes (Raynaud’s syndrome)
- stomach and intestinal problems (gastrointestinal and colonic ischemic events). Symptoms of gastrointestinal and colonic ischemic events include:
- o sudden or severe stomach pain
- o stomach pain after meals
- o weight loss
- o fever
- o nausea or vomiting
- o constipation or diarrhea
- o bloody diarrhea
-
problems with blood circulation to your legs and feet (peripheral vascular ischemia). Symptoms of peripheral vascular ischemia include:
- o cramping and pain in your legs or hips
- o feeling of heaviness or tightness in your leg muscles
- o burning or aching pain in your feet or toes while resting
- o numbness, tingling, or weakness in your legs
- o cold feeling or color changes in 1 or both legs or feet
- medication overuse headaches. Some people who use too many IMITREX injections may have worse headaches (medication overuse headache). If your headaches get worse, your healthcare provider may decide to stop your treatment with IMITREX.
- serotonin syndrome. Serotonin syndrome is a rare but serious problem that can happen in people using IMITREX, especially if IMITREX is used with anti‑depressant medicines called SSRIs or SNRIs.
-
Call your healthcare provider right away if you have any of the following symptoms of serotonin syndrome:
- o mental changes such as seeing things that are not there (hallucinations), agitation, or coma
- o fast heartbeat
- o changes in blood pressure
- o high body temperature
- o tight muscles
- o trouble walking
- hives (itchy bumps); swelling of your tongue, mouth or throat.
- seizures. Seizures have happened in people taking IMITREX who have never had seizures before. Talk with your healthcare provider about your chance of having seizures while you take IMITREX.
The most common side effects of IMITREX Injection include:
- pain or redness at your injection site
- tingling or numbness in your fingers or toes
- dizziness
- warm, hot, burning feeling to your face (flushing)
- discomfort or stiffness in your neck
- feeling weak, drowsy, or tired
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of IMITREX. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store IMITREX injection?
- Store IMITREX between 36°F to 86°F (2°C to 30°C).
- Store your medicine away from light.
- Keep your medicine in the packaging or carrying case provided with it.
Keep IMITREX and all medicines out of the reach of children.
General information about the safe and effective use of IMITREX
Medicines are sometimes prescribed for purposes other than those listed in Patient Information leaflets. Do not use IMITREX for a condition for which it was not prescribed. Do not give IMITREX to other people, even if they have the same symptoms you have. It may harm them.
This Patient Information leaflet summarizes the most important information about IMITREX. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about IMITREX that is written for healthcare professionals.
For more information, go to www.gsk.com or call 1-888-825-5249.
What are the ingredients in IMITREX injection?
Active ingredient: sumatriptan succinate
Inactive ingredients: sodium chloride, water for injection
IMITREX and AMERGE are trademarks owned by or licensed to the GSK group of companies. The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to the GSK group of companies. The makers of these brands are not affiliated with and do not endorse the GSK group of companies or its products.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2018 GSK group of companies or its licensor.
IMJ:7PPI
- This Patient Information has been approved by the U.S. Food and Drug Administration. Revised July 2018
Read this Instructions for Use before you start to use the IMITREX STATdose System. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or treatment. You and your healthcare provider should talk about IMITREX Injection when you start taking it and at regular checkups.
Keep the IMITREX STATdose System out of the reach of children.
Before you use the IMITREX STATdose System
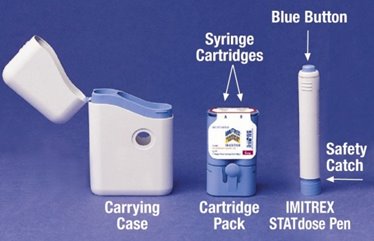
When you first open the IMITREX STATdose System box, the Cartridge Pack and the IMITREX STATdose Pen are already in the Carrying Case for your convenience.
The grey and blue Carrying Case is used for storing the unloaded Pen and the Cartridge Pack when they are not being used.
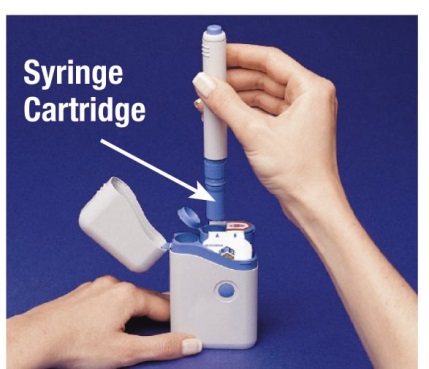
- The Cartridge Pack holds 2 individually sealed Syringe Cartridges. Each Syringe Cartridge holds 1 dose of IMITREX (sumatriptan succinate) Injection.
- The Cartridge Pack for the 4-mg strength of this medicine is yellow.
- The Cartridge Pack for the 6-mg strength is blue (as shown).
- Refill Cartridge Packs are available.
Important Things to Know About Your IMITREX STATdose System
- The needle shield of the prefilled syringe contains dry natural rubber, which is made from latex. This is a part you cannot usually see, but it could still cause an allergic reaction. Tell your healthcare provider if you are allergic to latex.
- Before you use IMITREX STATdose System your healthcare provider should show you or your caregiver how to give an injection the right way.
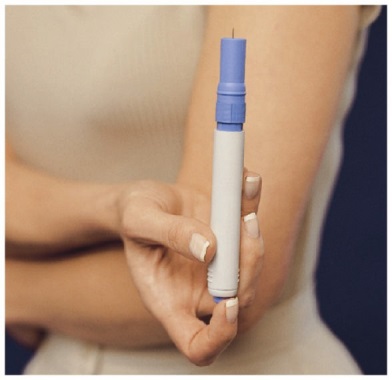
- The Pen is used to automatically inject 1 dose of medicine from a Syringe Cartridge.
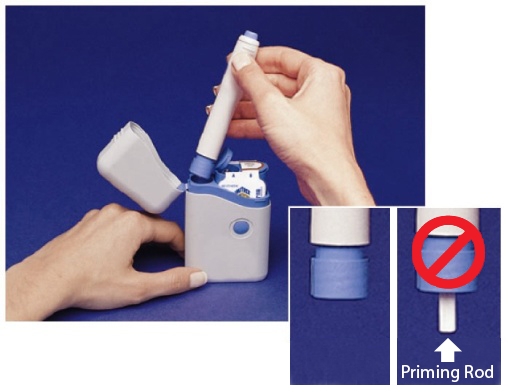
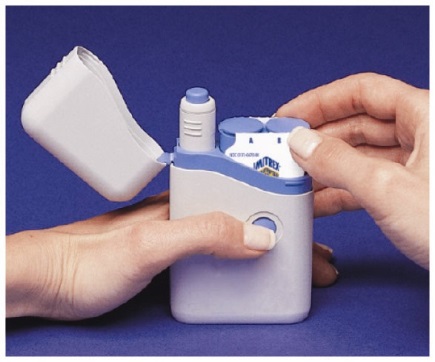
- Before you load a Syringe Cartridge, always check to make sure that the white Priming Rod is not sticking out from the end of the Pen (as shown below in Figure B). If it is sticking out, you will lose that dose.
- Do not touch the Blue Button until you have pressed the Pen firmly against your skin to give a dose.
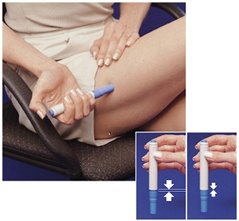
- The Pen will only work when the Safety Catch is released. To release the Safety Catch, you must press the Pen firmly against your skin until the grey part of the barrel slides against the blue part and it cannot be pressed any further. The grey part of the barrel must stay in contact with the blue part while you inject your medicine.
- When you inject the dose, make sure the Pen stays in contact with your skin during the injection. It is important to hold the Pen against the skin for at least 5 seconds.
- After each use the Pen must be put back into the Carrying Case to reset the white Priming Rod before the next use.
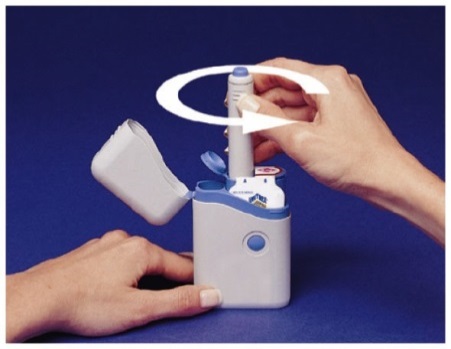
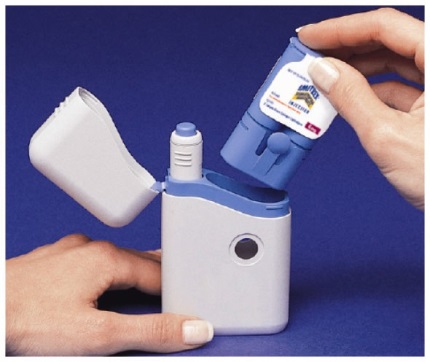
How to load the IMITREX STATdose Pen
Do not load the Pen until you are ready to give yourself an injection.
Do not touch the Blue Button on top of the Pen (see Figure A)
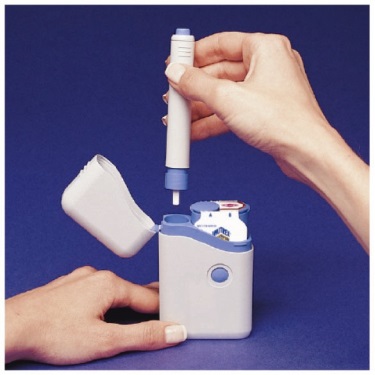
while you are loading the Pen.How to unload the IMITREX STATdose Pen after taking your medicine
- Right after you complete your injection with the Pen, you need to return the used Syringe Cartridge to the Cartridge Pack.
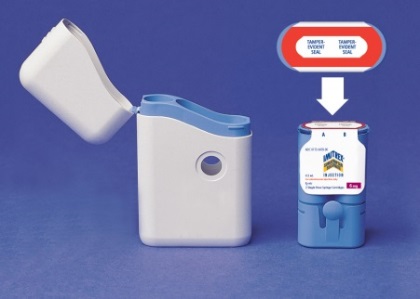
How to take out a used Cartridge Pack
After both Syringe Cartridges have been used, take the Cartridge Pack out of the Carrying Case. Do not reuse or recycle a Syringe Cartridge.
How to insert a new Cartridge Pack
How to dispose of your used Syringe Cartridge Pack
Put your used syringe cartridge pack in a FDA-cleared sharps disposal container right away after use (see Figure Q). Do not throw away (dispose of) loose needles and syringes in your household trash.
Figure Q
-
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out, upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and cartridges. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Trademarks are owned by or licensed to the GSK group of companies.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2018 GSK group of companies or its licensor.
July 2018
IMJ:8PIL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 0173-0739-02
IMITREX®
(SUMATRIPTAN SUCCINATE)
INJECTION
4 mg
0.5 mL
For subcutaneous injection only.
2 Single-Dose Syringe Cartridges
Rx only
- 10000000109572 Rev. 8/12
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 0173-0478-00
IMITREX®
(SUMATRIPTAN SUCCINATE)
INJECTION
6 mg
0.5 mL
For subcutaneous injection only.
Rx only
2 Single-Dose Syringe Cartridges
- 10000000109517 Rev. 9/12
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 0173-0449-02
IMITREX®
(SUMATRIPTAN SUCCINATE)
INJECTION
6 mg
0.5 mL
For subcutaneous injection only.
Single-dose vial. Discard unused portion.
Rx only
5 Single-Dose Vials
Made in Spain
©2017 the GSK group of companies
- 10000000146845 Rev. 6/17
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC: 0173-0479-00
IMITREX
STATdose SYSTEM
6 mg
This carton contains:
- Imitrex
(sumatriptan succinate)
Injection
2 prefilled 0.5-mL syringe cartridges, each containing 6 mg of sumatriptan for subcutaneous injection only
- 1 Imitrex STATdose Pen
- 1 Carrying Case
- Instructions for Use/Information for the Patient
- Prescribing Information
This Product Contains Dry Natural Rubber
Rx only
Do not use if tamper-evident label is broken or missing.
Made in India
©2018 the GSK group of companies
- 62000000028442 Rev. 12/18
-
INGREDIENTS AND APPEARANCE
IMITREX
sumatriptan succinate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0173-0739 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUMATRIPTAN SUCCINATE (UNII: J8BDZ68989) (SUMATRIPTAN - UNII:8R78F6L9VO) SUMATRIPTAN 4 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.8 mg in 0.5 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (clear, colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0173-0739-00 2 in 1 PACKAGE 04/06/2006 1 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC: 0173-0739-02 2 in 1 CARTON 04/06/2006 2 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020080 04/06/2006 IMITREX
sumatriptan succinate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0173-0479 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUMATRIPTAN SUCCINATE (UNII: J8BDZ68989) (SUMATRIPTAN - UNII:8R78F6L9VO) SUMATRIPTAN 6 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.5 mg in 0.5 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (clear, colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0173-0479-00 2 in 1 PACKAGE 01/23/1997 1 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020080 01/23/1997 IMITREX
sumatriptan succinate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0173-0478 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUMATRIPTAN SUCCINATE (UNII: J8BDZ68989) (SUMATRIPTAN - UNII:8R78F6L9VO) SUMATRIPTAN 6 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.5 mg in 0.5 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (clear, colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0173-0478-00 2 in 1 CARTON 01/27/1997 1 0.5 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020080 01/27/1997 IMITREX
sumatriptan succinate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0173-0449 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUMATRIPTAN SUCCINATE (UNII: J8BDZ68989) (SUMATRIPTAN - UNII:8R78F6L9VO) SUMATRIPTAN 6 mg in 0.5 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) 3.5 mg in 0.5 mL WATER (UNII: 059QF0KO0R) Product Characteristics Color YELLOW (clear, colorless to pale yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0173-0449-02 5 in 1 CARTON 02/23/1993 1 0.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020080 02/23/1993 Labeler - GlaxoSmithKline LLC (167380711)
Trademark Results [IMITREX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 IMITREX 98398968 not registered Live/Pending |
Glaxo Group Limited 2024-02-09 |
 IMITREX 87686176 5438412 Live/Registered |
Glaxo Group Limited 2017-11-15 |
 IMITREX 74590264 1946573 Dead/Cancelled |
GLAXO GROUP LIMITED 1994-10-25 |
 IMITREX 74164514 not registered Dead/Abandoned |
GLAXO GROUP LIMITED 1991-05-07 |
 IMITREX 74081658 1787324 Live/Registered |
GLAXO GROUP LIMITED 1990-07-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.