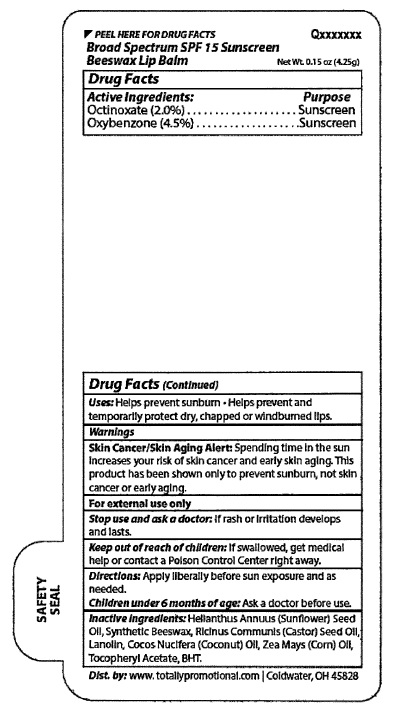
Broad Spectrum SPF 15 Sunscreen
Beeswax Lip Balm SPF 15 by
Drug Labeling and Warnings
Beeswax Lip Balm SPF 15 by is a Otc medication manufactured, distributed, or labeled by Casad Company Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BEESWAX LIP BALM SPF 15- lip balm stick
Casad Company Inc.
----------
Broad Spectrum SPF 15 Sunscreen
Warnings
Skin Cancer/Skin Aging Alert: SpendIng tIme In the sun
increases your risk of skin cancer and early skin aging. This
product has been shown only to prevent sunburn, not skin
cancer or early aging.
For external use only
Stop use and ask a doctor: lf rash or irritation develops
and lasts.
Keep out of reach of children: If swallowed, get medilcal
help or contact a Poison Control center right away.
Directlons: Apply liberally before sun exposure and as
needed.
Children unde 6 months of age: Ask a doctor before use.
| BEESWAX LIP BALM SPF 15
lip balm stick |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Casad Company Inc. (877343673) |
| Registrant - Casad Company Inc. (877343673) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.