BDR RE-CHARGE N HYDRO SERUM MAXIMUM SKIN ENERGIZER- panthenol liquid
bdr Re-charge N hydro serum maximum skin energizer by
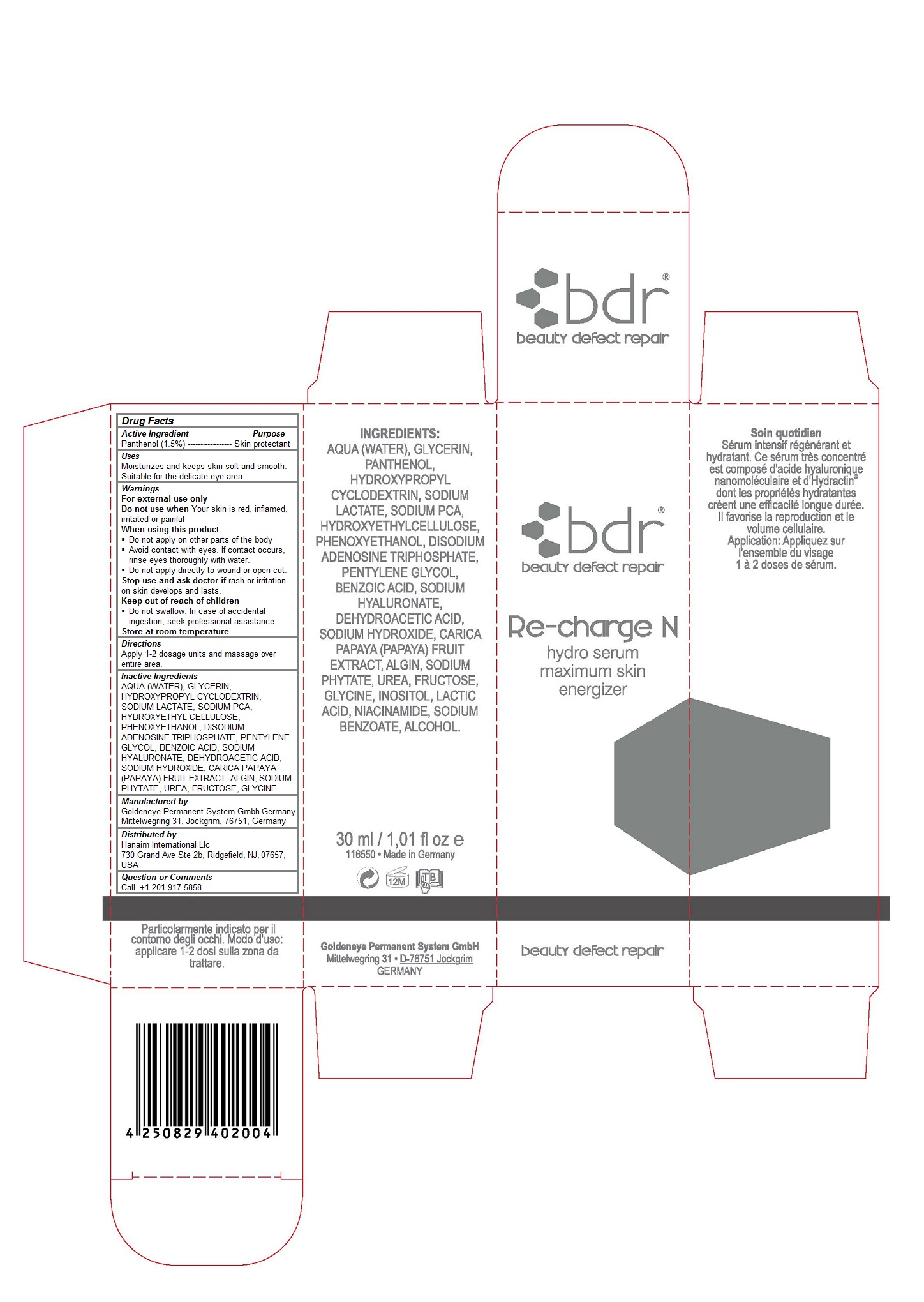
Drug Labeling and Warnings
bdr Re-charge N hydro serum maximum skin energizer by is a Otc medication manufactured, distributed, or labeled by Goldeneye Permanent System Gmbh Germany, Hanaim International Llc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Purpose
- Keep out of reach of children
-
Warnings
For external use only
Do not use when Your skin is red, inflamed, irritated or painful
When using this product
Do not apply on other parts of the body
Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Do not apply directly to wound or open cut.
Stop use and ask doctor if rash or irritation on skin develops and lasts.
Keep out of reach of children
Do not swallow. In case of accidental ingestion, seek professional assistance.
Store at room temperature - Uses
- Directions
-
Inactive Ingredients
AQUA (WATER), GLYCERIN, HYDROXYPROPYL CYCLODEXTRIN, SODIUM LACTATE, SODIUM PCA, HYDROXYETHYL CELLULOSE, PHENOXYETHANOL, DISODIUM ADENOSINE TRIPHOSPHATE, PENTYLENE GLYCOL, BENZOIC ACID, SODIUM HYALURONATE, DEHYDROACETIC ACID, SODIUM HYDROXIDE, CARICA PAPAYA (PAPAYA) FRUIT EXTRACT, ALGIN, SODIUM PHYTATE, UREA, FRUCTOSE, GLYCINE
- bdr Re-charge N hydro serum maximum skin energizer
-
INGREDIENTS AND APPEARANCE
BDR RE-CHARGE N HYDRO SERUM MAXIMUM SKIN ENERGIZER
panthenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71056-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANTHENOL (UNII: WV9CM0O67Z) (PANTHENOL - UNII:WV9CM0O67Z) PANTHENOL 0.45 g in 30 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LACTATE (UNII: TU7HW0W0QT) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) HYDROXYETHYL CELLULOSE (100 MPA.S AT 2%) (UNII: R33S7TK2EP) PHENOXYETHANOL (UNII: HIE492ZZ3T) ADENOSINE TRIPHOSPHATE DISODIUM (UNII: 5L51B4DR1G) PENTYLENE GLYCOL (UNII: 50C1307PZG) BENZOIC ACID (UNII: 8SKN0B0MIM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DEHYDROACETIC ACID (UNII: 2KAG279R6R) SODIUM HYDROXIDE (UNII: 55X04QC32I) PAPAYA (UNII: KU94FIY6JB) SODIUM ALGINATE (UNII: C269C4G2ZQ) PHYTATE SODIUM (UNII: 88496G1ERL) UREA (UNII: 8W8T17847W) FRUCTOSE (UNII: 6YSS42VSEV) GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71056-105-02 1 in 1 PACKAGE 11/15/2016 1 NDC: 71056-105-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/15/2016 Labeler - Hanaim International Llc (070319425) Registrant - Hanaim International Llc (070319425) Establishment Name Address ID/FEI Business Operations Hanaim International Llc 070319425 relabel(71056-105) Establishment Name Address ID/FEI Business Operations Goldeneye Permanent System Gmbh Germany 329178144 manufacture(71056-105)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.