Enokon Pain relief patch by Henan Enokon Medical Instrument Co Ltd. Complete
Enokon Pain relief patch by
Drug Labeling and Warnings
Enokon Pain relief patch by is a Otc medication manufactured, distributed, or labeled by Henan Enokon Medical Instrument Co Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
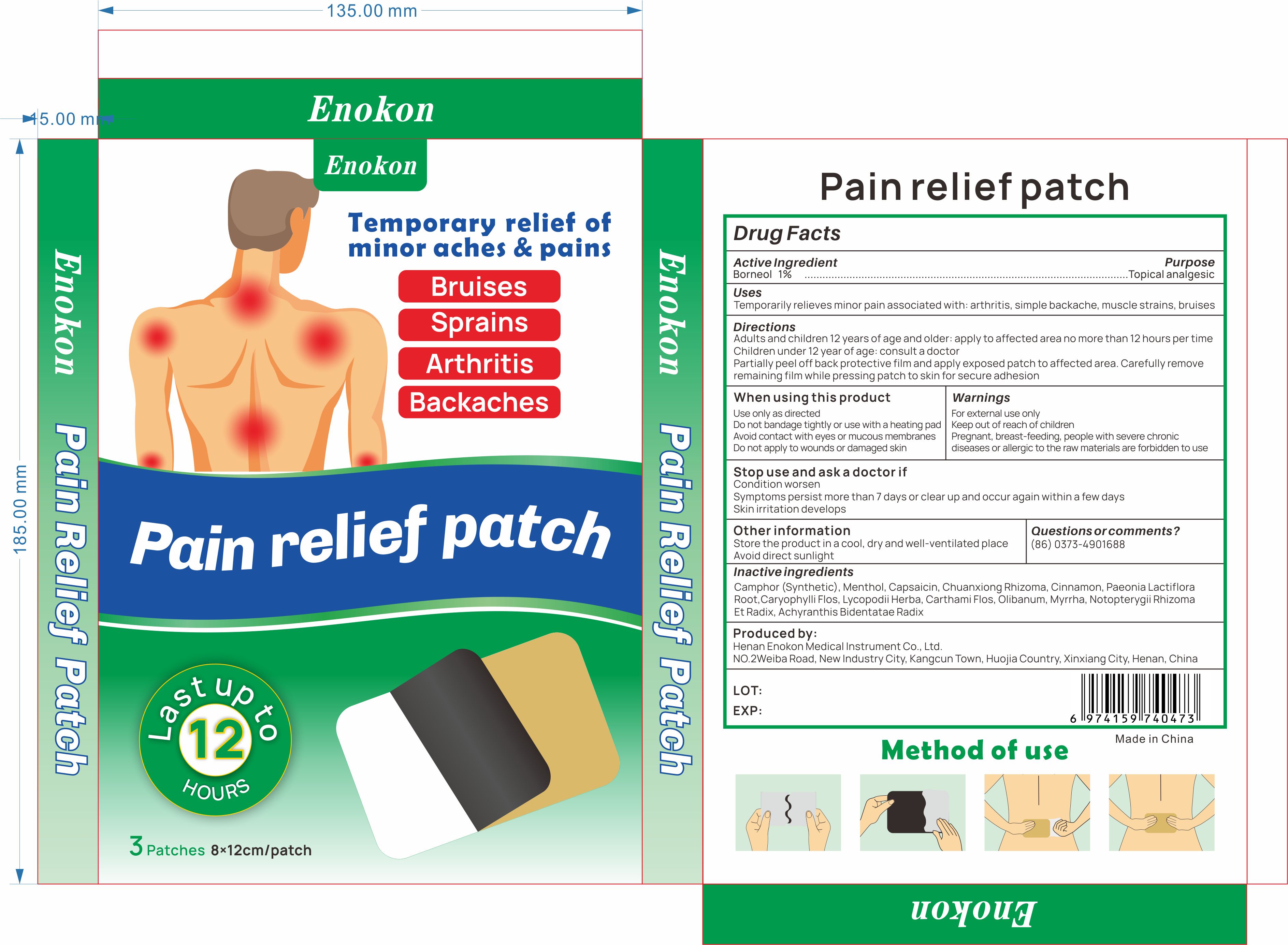
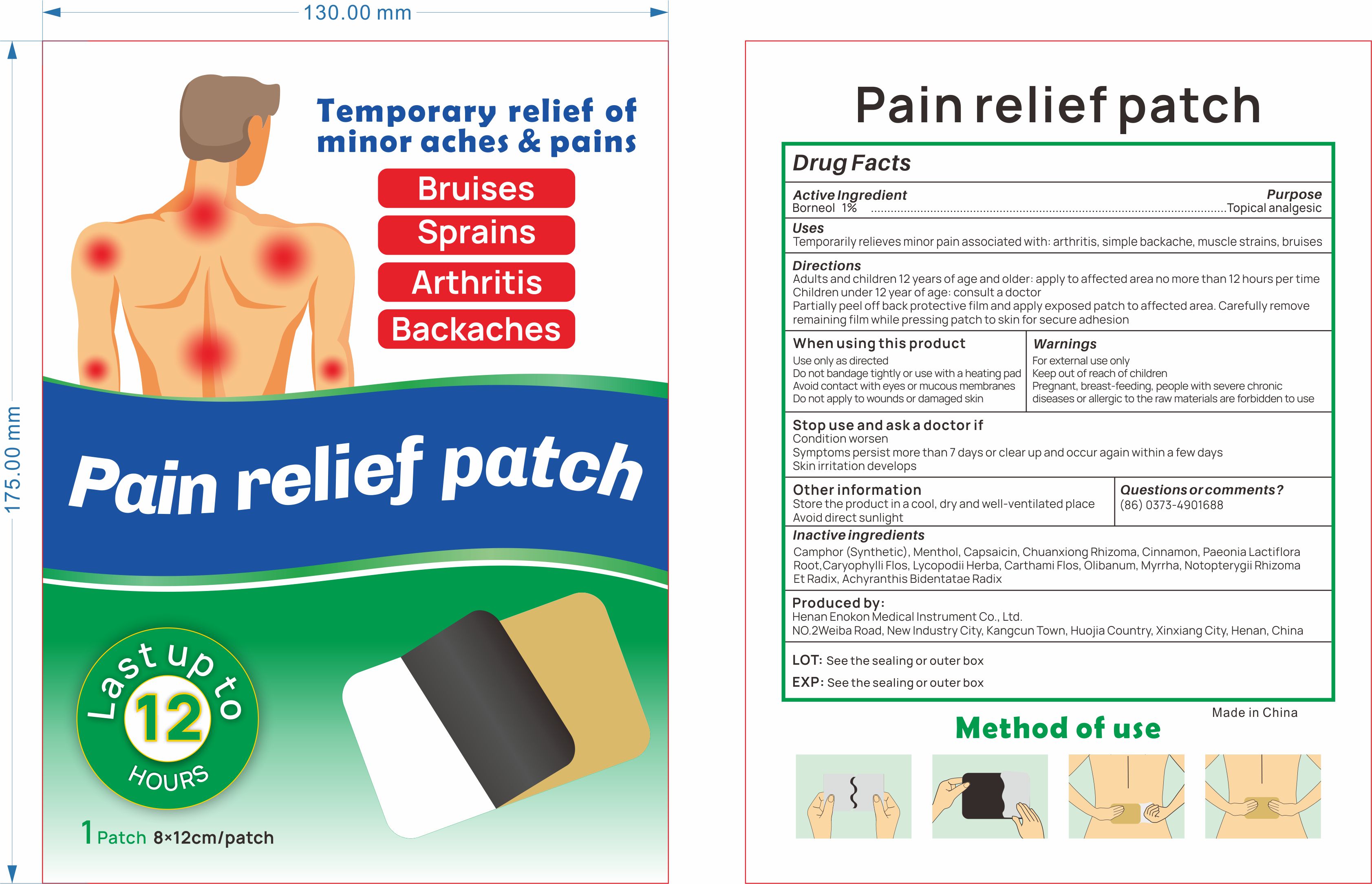
ENOKON PAIN RELIEF PATCH- pain relief patch patch
Henan Enokon Medical Instrument Co Ltd.
----------
Complete
Use
Temporarily relieves minor pain associated with: arthritis, simple backache, muscle strains, bruises
Warnings
For externaluse only Keep out ofreach of children Pregnant, breast-feeding, people with severe chronicdiseases or allergic to the raw materials are forbidden to use
When Using
Use only as directed
Do not bandage tightly or use with a heating pad Avoid contact with eyes or mucous membranes
Do not apply to wounds or damaged skin
Ask Doctor
Condition worsen
Symptoms persist more than 7 days or clear up and occur again within a few days
Skin irritation develops
Directions
Adults and children 12 years of age and older: apply to affected area no more than 12 hours per timeChildren under 12 vear ofage: consult a doctorPeel off back protective film and alian the stainless steel ball with the affected area and apply patch, pressingpatch to skin for secure adhesion
| ENOKON PAIN RELIEF PATCH
pain relief patch patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Henan Enokon Medical Instrument Co Ltd. (701730676) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Henan Enokon Medical Instrument Co Ltd. | 701730676 | manufacture(83559-002) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.