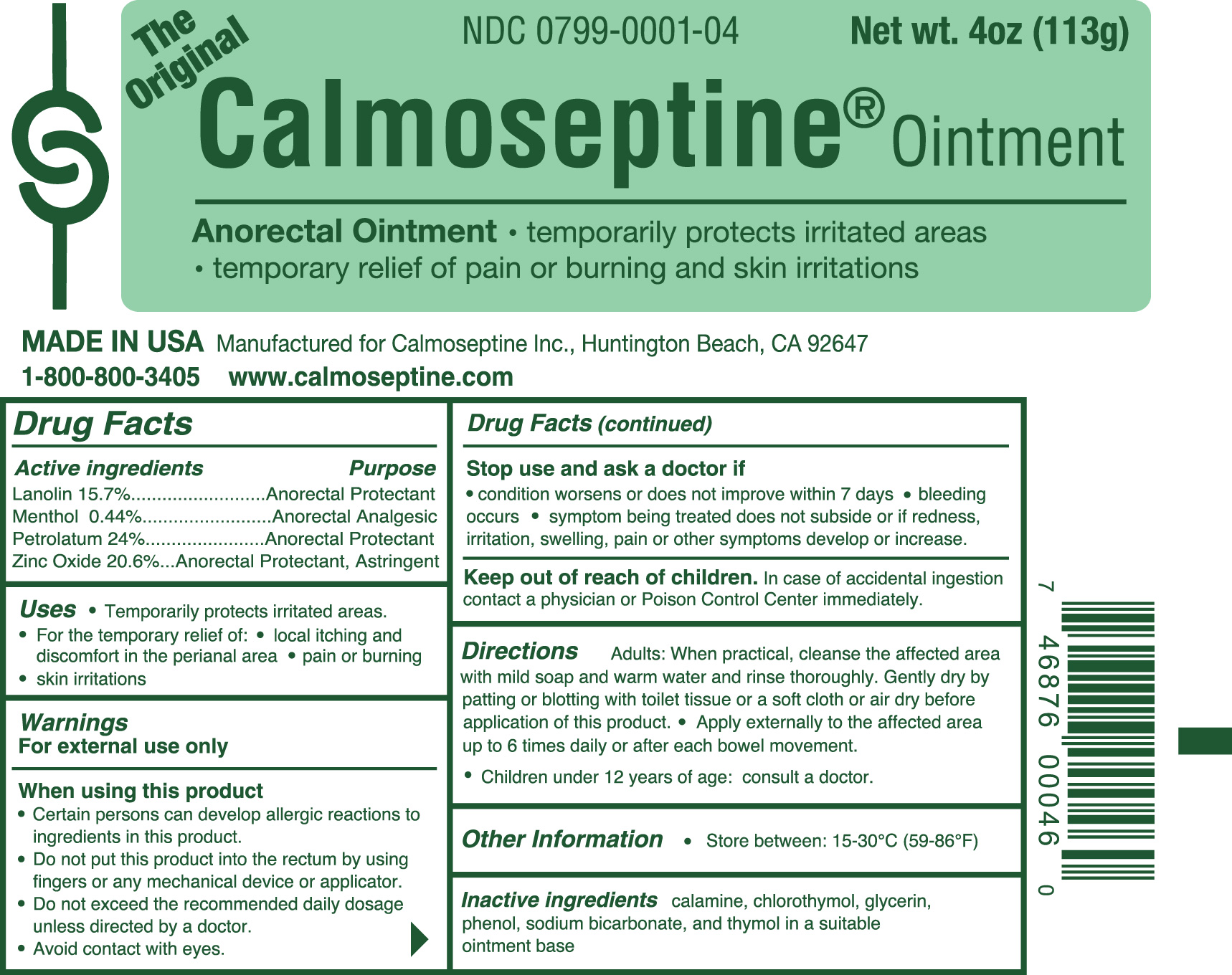
CALMOSEPTINE- anorectal ointment ointment
Calmoseptine by
Drug Labeling and Warnings
Calmoseptine by is a Otc medication manufactured, distributed, or labeled by Calmoseptine Inc. , Calmo Manufacturing Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Uses
-
Warnings
For external use only
When using this product
* Certain persons can develop allergic reactions to ingredients in this product.
* Do not put this product into the rectum by using fingers or any mechanical device or applicator.
* Do not exceed the recommended daily dosage unless directed by a doctor.
* Avoid contact with eyes. - Stop use and ask doctor if
- Keep out of reach of children.
-
Directions
Adults: When practical, cleanse the affected area with mild soap and warm water and rinse thoroughly.
Gently dry by patting or blotting with toilet tissue or a soft cloth or air dry before application of this product.
Apply externally to the affected area up to 6 times daily or after each bowel movement.
Children under 12 years of age: consult a doctor. - Inactive Ingredients:
- Calmoseptine Ointment
-
INGREDIENTS AND APPEARANCE
CALMOSEPTINE
anorectal ointment ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0799-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANOLIN (UNII: 7EV65EAW6H) (LANOLIN - UNII:7EV65EAW6H) LANOLIN 15.7 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.44 g in 100 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 24 g in 100 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 20.6 g in 100 g Inactive Ingredients Ingredient Name Strength FERRIC OXIDE RED (UNII: 1K09F3G675) CHLOROTHYMOL (UNII: LJ25TI0CVT) GLYCERIN (UNII: PDC6A3C0OX) PHENOL (UNII: 339NCG44TV) SODIUM BICARBONATE (UNII: 8MDF5V39QO) THYMOL (UNII: 3J50XA376E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0799-0001-02 71 g in 1 TUBE; Type 0: Not a Combination Product 01/04/1999 2 NDC: 0799-0001-03 71 g in 1 JAR; Type 0: Not a Combination Product 08/01/1950 3 NDC: 0799-0001-04 113 g in 1 TUBE; Type 0: Not a Combination Product 08/01/1988 4 NDC: 0799-0001-05 3.5 g in 1 PACKET; Type 0: Not a Combination Product 06/01/2001 5 NDC: 0799-0001-06 20 g in 1 TUBE; Type 0: Not a Combination Product 07/03/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 08/01/1950 Labeler - Calmoseptine Inc. (783409394) Establishment Name Address ID/FEI Business Operations Calmo Manufacturing Inc. 015850149 manufacture(0799-0001) , pack(0799-0001) , label(0799-0001)
Trademark Results [Calmoseptine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CALMOSEPTINE 73754242 1632114 Dead/Cancelled |
PERRY LABORATORIES INC. 1988-09-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.