PRADALEX- pradofloxacin injection, solution
Pradalex by
Drug Labeling and Warnings
Pradalex by is a Animal medication manufactured, distributed, or labeled by Elanco US Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
CAUTION
Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Federal law prohibits the extra-label use of this drug in food-producing animals.
To ensure responsible antimicrobial drug use, use of pradofloxacin should be limited to treatment of bovine respiratory disease (BRD) in cattle and treatment of swine respiratory disease (SRD) in swine only after consideration of other non-fluoroquinolone therapeutic options.
-
PRODUCT DESCRIPTION
Pradalex (pradofloxacin injection) is a sterile, ready-to-use injectable antimicrobial solution that contains pradofloxacin, a broad-spectrum fluoroquinolone antimicrobial agent.
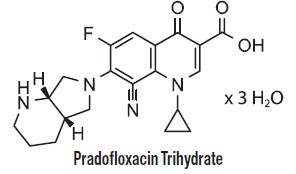
Each mL of Pradalex contains 227 mg pradofloxacin trihydrate; equivalent to 200 mg of pradofloxacin. Excipients are citric acid (antioxidant) 1 mg, gluconolactone (for pH adjustment) 77 mg, and water for injection q.s. Pradofloxacin is a fluoroquinolone antimicrobial and belongs to the class of quinoline carboxylic acid derivatives. Its chemical name is 8-cyano-1-cyclopropyl-6-fluoro-7-[(4aS,7aS)-octahydro-6Hpyrrolo[3,4-b]pyridin-6-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid.
-
INDICATIONS
Cattle: Pradalex is indicated for the treatment of BRD associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis in cattle intended for slaughter (beef calves 2 months of age and older, growing beef steers, growing beef heifers, and beef bulls intended for slaughter), and in cattle intended for breeding less than 1 year of age (replacement beef and dairy heifers less than 1 year of age and beef and dairy bulls less than 1 year of age). Not for use in cattle intended for breeding 1 year of age and older (replacement beef and dairy heifers 1 year of age and older, beef and dairy bulls 1 year of age and older, and beef and dairy cows), beef calves less than 2 months of age, dairy calves, and veal calves.
Swine: Pradalex is indicated for the treatment of SRD associated with Bordetella bronchiseptica, Glaesserella (Haemophilus) parasuis, Pasteurella multocida, Streptococcus suis, and Mycoplasma hyopneumoniae in weaned swine intended for slaughter (nursery, growing, and finishing swine, boars intended for slaughter, barrows, gilts intended for slaughter, and sows intended for slaughter).
Not for use in swine intended for breeding (boars intended for breeding, replacement gilts, and sows intended for breeding) and in nursing piglets.
-
DOSAGE AND ADMINISTRATION
Cattle: Administer once as a subcutaneous injection at a dosage of 10 mg/kg (2.3 mL/100 lb) body weight. Do not inject more than 15 mL per subcutaneous injection site.
Table 1. Pradalex Dose Guide for Cattle (2.3 mL/100 lbs)
Weight (lb)
Dose Volume (mL)
100
2.3
200
4.6
300
6.9
400
9.2
500
11.5
600
13.8
700
16.1
800
18.4
900
20.7
Swine: Administer once as an intramuscular injection in the neck at a dosage of 7.5 mg/kg (1.7 mL/100 lb) body weight. Do not inject more than 5 mL per intramuscular injection site.
Table 2. Pradalex Dose Guide for Swine (1.7 mL/100 lbs)
Weight (lb)
Dose Volume (mL)
15
0.3
30
0.5
50
0.9
100
1.7
150
2.6
200
3.4
250
4.3
Dilution of Pradalex: Pradalex may be diluted with sterile water, sterile saline (0.9%), or 5% dextrose (D5W) prior to injection. The diluted product should be used within 24 hours. Store diluted solution in amber glass bottles between 25-40°C (77-104°F).
Table 3. Dilution Guide for Swine*
*For 1 mL dose volume from diluted solution **Pradalex can be diluted with sterile water, sterile saline (0.9%), or 5% dextrose (D5W) for injection Swine Weight
mL of Pradalex
mL of diluent**
Number of doses
5 lb
8.5 mL
91.5 mL
100
10 lb
17 mL
83 mL
100
15 lb
25.6 mL
74.4 mL
100
20 lb
34.1 mL
65.9 mL
100
25 lb
42.6 mL
57.4 mL
100
30 lb
51.1 mL
48.9 mL
100
35 lb
59.7 mL
40.3 mL
100
40 lb
68.2 mL
31.8 mL
100
45 lb
76.7 mL
23.3 mL
100
50 lb
85.2 mL
14.8 mL
100
Use bottle within 6 months of first puncture. When administering from the 250 mL bottle, puncture a maximum of 120 times. If more than 120 punctures are anticipated, the use of multi-dosing equipment is recommended. When using a draw-off spike or needle with bore diameter larger than 16-gauge, discard any product remaining in the vial immediately after use.
-
WITHDRAWAL PERIODS and RESIDUE WARNINGS
Cattle intended for human consumption must not be slaughtered within 4 days of treatment. Swine intended for human consumption must not be slaughtered within 2 days of treatment. Not for use in female dairy cattle 1 year of age and older, including dry dairy cows; use in these cattle may cause drug residues in milk and/or in calves born to these cows. Not for use in beef calves less than 2 months of age, dairy calves, and veal calves; a withdrawal period has not been established for this product in pre-ruminating calves.
-
WARNINGS
USER SAFETY WARNINGS
Not for use in humans. Keep out of reach of children. Avoid contact with eyes and skin. In case of ocular contact, immediately remove contact lenses and flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water for at least 20 seconds. Consult a physician if irritation persists following ocular or dermal exposures, or in case of accidental ingestion. Individuals with a history of hypersensitivity to quinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight. Do not eat, drink or smoke while handling this product. To obtain a copy of the Safety Data Sheet, contact Elanco at 1-800-428-4441.
ANIMAL SAFETY WARNINGS
Not for use in animals intended for breeding because the effects of Pradalex on bovine and swine reproductive performance, pregnancy, and lactation have not been determined. Not for use in pre-ruminating calves or nursing piglets because safety and effectiveness has not been demonstrated. Swelling and inflammation may be seen at the injection site after administration. These local tissue reactions may persist beyond the slaughter withdrawal period and may result in trim loss of edible tissue at slaughter.
Quinolones should be used with caution in animals with known or suspected central nervous system (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation that may lead to convulsive seizures. Quinolones have been shown to produce erosions of cartilage of weightbearing joints and other signs of arthropathy in immature animals of various species. See Target Animal Safety section for additional information.
- ADVERSE REACTIONS
-
CONTACT INFORMATION
To report suspected adverse drug experiences, for technical assistance or to obtain a copy of the Safety Data Sheet, contact Elanco at 1-800-428-4441. For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Pradofloxacin is a synthetic fluoroquinolone antibacterial drug. Pradofloxacin acts via inhibition of DNA gyrase and topoisomerase IV enzymes in bacteria to inhibit DNA and RNA synthesis. It is bactericidal with a broad spectrum of activity. As a class, fluoroquinolones are considered concentration dependent antimicrobials. Pradofloxacin induces long post-antibiotic effects (PAE) and extended post-antibiotic sub-MIC effects (PA SME), both in aerobic and anaerobic bacteria.
Pharmacokinetics
Cattle: The pharmacokinetic parameters of pradofloxacin in Table 4 were determined from two studies following subcutaneous administration of pradofloxacin in 4- to-5-month-old weaned calves weighing 158 to 319 kg.
Pradofloxacin exposure (Cmax and AUC) was dose proportional over a 10 to 50 mg/kg dose range with no accumulation when administered once every 4 days over 8 days.
Pradofloxacin was excreted in both the urine and the feces, largely unchanged, with the majority of the administered dose being excreted in the first 24 hours post-dosing.
Swine: The pharmacokinetic parameters of pradofloxacin in Table 4 were determined following intramuscular administration of pradofloxacin in 18-day-old weaned pigs weighing 5.5 to 7.9 kg. Pradofloxacin exposure (Cmax and AUC) was dose proportional over a 7.5 to 37.5 mg/kg dose range with no accumulation when administered once every 2 days over 4 days.
Pradofloxacin was excreted in both the urine and the feces, largely unchanged, with approximately one-third of the administered dose being excreted in the first 24 hours post-dosing.
Table 4. Arithmetic mean (± standard deviation) plasma pradofloxacin pharmacokinetic parameters following the first of three administrations of Pradalex (pradofloxacin injection).
a Reported as: Median (range)
b N=11 due to inability to calculate half-life in 1 animal
Cmax = maximum concentration
Tmax = time to maximum concentration
AUClast = area under the curve from the time of dosing to the time of the last measurable concentration
t½ = half-lifePharmacokinetic
ParameterWeaned calves (N=12)
10 mg/kg BW SCWeaned pigs (N = 8)
7.5 mg/kg BW IMCmax (μg/mL)
1.9 ± 0.4
2.5 ± 1.9
Tmax (hours)a
1 (1 to 2)
0.75 (0.5 to 2)
AUClast (hr*μg/mL)
10.5 ± 1.2
26.2 ± 3.7
t1/2 (hours)
2.8 ± 0.4b
8.5 ± 2.6
-
MICROBIOLOGY
Cattle: The minimal inhibitory concentrations (MICs) of pradofloxacin were determined for isolates of Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis which were obtained from cattle enrolled in the 2015 BRD field study conducted in the U.S. MIC determinations were completed using Clinical and Laboratory Standards Institute (CLSI) standard methods except for M. bovis. The methods and quality control performance standards for M. bovis were validated with a multi-center laboratory study. The results are shown below in Table 5.
Table 5. Pradofloxacin MIC values* of BRD pathogens isolated from the 2015 field study.
* The correlation between in vitro susceptibility data and clinical effectiveness is unknown. ** The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively. BRD Pathogens
No. of
IsolatesMIC50**
(μg/mL)MIC90**
(μg/mL)MIC range
(μg/mL)M. haemolytica
365
0.008
2
0.001 to 2
P. multocida
248
0.008
0.015
0.001 to 0.12
H. somni
106
0.015
0.015
0.015 to 0.25
M. bovis
159
0.12
0.5
0.002 to 1
Swine: The MICs of pradofloxacin were determined for isolates of Bordetella bronchiseptica, Glaesserella (Haemophilus) parasuis, Pasteurella multocida and Streptococcus suis which were obtained from swine enrolled in the 2017 SRD field study conducted in the U.S. MIC determinations were completed using CLSI standard methods except for G. parasuis. The methods for G. parasuis were validated with a multi-center laboratory study. The results are shown below in Table 6.
Table 6. Pradofloxacin MIC values* of SRD pathogens isolated from the 2017 field study.
* The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
** The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.SRD Pathogens
No. of
IsolatesMIC50**
(μg/mL)MIC90**
(μg/mL)MIC range (μg/mL)
B. bronchiseptica
111
0.12
0.12
0.12 to 0.25
G. parasuis
93
0.001
0.004
0.00025 to 0.008
P. multocida
99
0.004
0.008
0.004 to 0.008
S. suis
212
0.06
0.25
0.015 to 4
-
SUMMARY OF SAFETY AND EFFECTIVENESS
EFFECTIVENESS
Cattle: The effectiveness of Pradalex for the treatment of BRD associated with Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis was demonstrated in a multi-site natural infection field study conducted in the U.S. A total of 630 commercial, mixed-breed male and female calves with clinical BRD were enrolled. Calves were administered a single subcutaneous dose of either Pradalex at 10 mg/kg body weight or an equivalent volume of sterile saline. Calves were evaluated for clinical success on Day 10. The success rate of Pradalex-treated calves (49.7%) was statistically significantly different (p = 0.0089) and numerically greater than that of saline-treated calves (25.6%) (based on back-transformed least squares means). No adverse events associated with Pradalex administration were reported in the study.
Swine: The effectiveness of Pradalex for the treatment of SRD associated with Bordetella bronchiseptica, Glaesserella (Haemophilus) parasuis, Pasteurella multocida, Streptococcus suis, and Mycoplasma hyopneumoniae was demonstrated in a multi-site, natural infection field study conducted in the U.S. A total of 1,200 castrated male and female growing pigs with clinical SRD were enrolled. At enrollment, pigs were administered a single intramuscular dose of either Pradalex at 7.5 mg/kg body weight, or an equivalent volume of sterile saline. Pigs were evaluated for clinical success on Day 7. The success rate of Pradalex-treated pigs (45.2%) was statistically significantly different (p=0.0274) and numerically greater than that of the saline-treated pigs (34.2%) (based on least squares means). No adverse events associated with Pradalex administration were reported in the study.
A total of 72 castrated male and female growing pigs were enrolled in an M. hyopneumoniae-induced challenge model study. Pigs were inoculated with a field strain of M. hyopneumoniae once daily for three consecutive days. Three days after the final inoculation, pigs were administered a single intramuscular dose of either Pradalex at 7.5 mg/kg body weight, or an equivalent volume of sterile saline. Pigs were euthanized and necropsied on Day 10. There was a significant difference (p=0.0002) in the mean total lung lesion score in favor of Pradalex-treated pigs (11.7%) compared to the saline-treated pigs (33.1%).
Cattle: Pradalex was evaluated in a margin of safety study with 32 healthy, weaned calves. Calves were randomized to four treatment groups: 0X (saline control), 1X (10 mg pradofloxacin/kg), 3X (30 mg pradofloxacin/kg) and 5X (50 mg pradofloxacin/kg). Calves were administered subcutaneous doses on Days 0, 4, and 8. All calves remained clinically normal throughout the in-life study and survived until scheduled necropsy on Day 9. Injection site swelling was noted in the 1X and 5X groups. Neutrophil counts, monocyte counts, and creatine kinase levels were generally higher in the treated groups, and this was attributed to inflammation and tissue damage at the injection sites. At necropsy, injection site lesions consisting of discoloration and edema, with microscopically visible hemorrhage, inflammation, and necrosis were reported in most treated animals. No signs of fluoroquinolone-induced arthropathy were reported. No other clinically significant adverse effects related to Pradalex were reported.
Pradalex was also evaluated in a margin of safety study focusing on pathologic changes to the testes and epididymides in 16 healthy, weaned bull calves. Calves were randomized to four treatment groups: 0X (saline control), 1X (10 mg pradofloxacin/kg), 3X (30 mg pradofloxacin/kg) and 5X (50 mg pradofloxacin/kg). Calves were administered subcutaneous doses on Days 0, 4, and 8. All calves remained clinically normal throughout the study and survived until scheduled castration on Day 9. Injection site swelling was noted in the 1X, 3X, and 5X groups. In the 1X group, 3 of 4 calves developed mild to moderate subcutaneous swellings, one resolved by 6 hours post-treatment and two were not resolved by the end of the study. No abnormal macroscopic or microscopic pathology in the testes or epididymides was reported.
Swine: Pradalex was evaluated in a margin of safety study with 32 healthy, weaned, crossbred pigs. Pigs were randomized to four treatment groups: 0X (saline control), 1X (7.5 mg pradofloxacin/kg), 3X (22.5 mg pradofloxacin/kg) and 5X (37.3mg pradofloxacin/kg). Pigs were administered intramuscular doses on Days 0, 2, and 4. All pigs remained clinically normal throughout the in-life study and all pigs survived until scheduled necropsy on Day 11. Aspartate aminotransferase (AST) and creatine phosphokinase (CK) showed elevations and statistically significant treatment by day interactions in Pradalex-treated pigs. These were attributed to inflammation and tissue damage at the injection sites and the values returned to normal by Day 10. There were no other clinically relevant effects on the remaining clinical pathology results. At necropsy, the only macroscopic and microscopic lesions attributable to Pradalex were related to tissue injury at the intramuscular injection sites. No signs of fluoroquinolone-induced arthropathy were reported. No other clinically significant adverse effects related to Pradalex were reported.
-
STORAGE CONDITIONS
Protect from direct sunlight. Do not refrigerate or freeze. Store at 25ºC (77ºF), excursions permitted up to 40ºC (104ºF) and down to -20ºC (-4ºF).
See in-use instructions provided in the Dosage and Administration section.
-
HOW SUPPLIED
200 mg/mL 250 mL bottles
200 mg/mL 100 mL bottlesPradalex is protected by one or more U.S. patents: see patent information at http://www.elancopatents.com
Approved by FDA under NADA # 141-550
Pradalex, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2025 Elanco or its affiliates
Product of Germany
Manufactured for: Elanco US Inc. Indianapolis, IN 46221
Revision August 2025
ElancoTM
PA600570X
-
Principal Display Panel – 100 mL Labels
Elanco™
Pradalex™
(pradofloxacin injection)Injectable Solution
Antimicrobial200 mg of pradofloxacin/mL
Net Contents: 100 mL
Approved by FDA under NADA # 141-550
Elanco
Pradalex™
(pradofloxacin injection)Injectable Solution
Antimicrobial
200 mg of pradofloxacin/mLNet Contents: 100 mL Approved by FDA under NADA # 141-550
-
Principal Display Panel – 250 mL Labels
Elanco™
Pradalex™
(pradofloxacin injection)Injectable Solution
Antimicrobial200 mg of pradofloxacin/mL
Net Contents: 250 mL
Approved by FDA under NADA # 141-550
Elanco™
Pradalex™
(pradofloxacin injection)Injectable Solution
Antimicrobial
200 mg of pradofloxacin/mLNet Contents: 250 mL
Approved by FDA under NADA # 141-550
-
INGREDIENTS AND APPEARANCE
PRADALEX
pradofloxacin injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-8527 Route of Administration SUBCUTANEOUS, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRADOFLOXACIN TRIHYDRATE (UNII: T9J58ERF3L) (PRADOFLOXACIN - UNII:6O0T5E048I) PRADOFLOXACIN 200 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-8527-1 1 in 1 CARTON 1 100 mL in 1 VIAL 2 NDC: 58198-8527-2 1 in 1 CARTON 2 250 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141550 04/09/2024 Labeler - Elanco US Inc. (966985624)
Trademark Results [Pradalex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRADALEX 98256393 not registered Live/Pending |
Elanco Animal Health GmbH 2023-11-06 |
 PRADALEX 88703804 not registered Live/Pending |
Bayer Intellectual Property GmbH 2019-11-22 |
 PRADALEX 87046382 not registered Live/Pending |
Bayer Intellectual Property GmbH 2016-05-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.