Prequel by The Center Brands, LLC DRUG FACTS
Prequel by
Drug Labeling and Warnings
Prequel by is a Otc medication manufactured, distributed, or labeled by The Center Brands, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
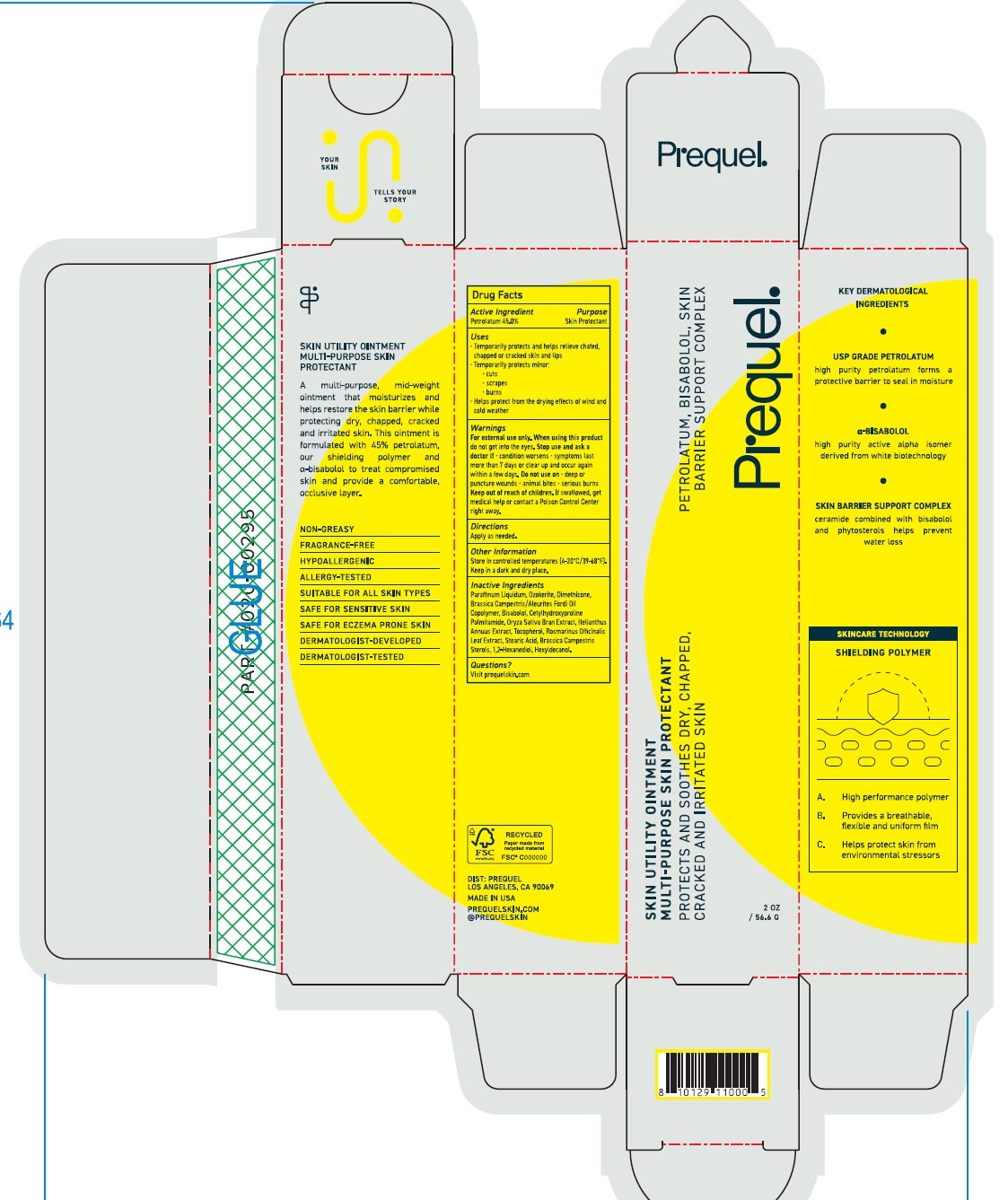
PREQUEL- multi purpose skin protectant ointment
The Center Brands, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
Uses
Temporarily protects and helps releive chafed, chapped or craked skin and lips
Temporarily protects minor:
cuts
scrapes
burns
Helps protect form the drying effects of wind and cold weather
Warnings
Fox external use only.
When using this product do not get into eyes
Stop use and ask a doctor if
- condition worsens
- symptoms last more than 7 days or clear up and occur again within a few days. Do not use on deep or puncture woulds animal bites serious burns.
Keep out of reach of children. If swalled, get medical help or contact a Poison Control Center right away.
Keep out of reach of children. If swalled, get medical help or contact a Poison Control Center right away.
Inactive Ingredients
Parrafinum Liquidum, Ozokerite, Dimethicone, Brassica Campestric/Aleuites Fordi Oil Copolymer, Bisabolol, Cetylhydroxyproline Palmitamide, Oryza Sativa Bran Extract, Helianthus Annuus Extract, Tocopherol, Rosmarinus Officinalis Leaf Extract, Stearic Acid, Brassica Campestris Sterols, 1,2-Hexanediol, Hexyldecanol
| PREQUEL
multi purpose skin protectant ointment |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - The Center Brands, LLC (076228814) |
Trademark Results [Prequel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PREQUEL 97623497 not registered Live/Pending |
The Center Brands LLC 2022-10-07 |
 PREQUEL 97466154 not registered Live/Pending |
Trilogy Enterprises, Inc. 2022-06-20 |
 PREQUEL 97021638 not registered Live/Pending |
Vigo Engineering Inc. 2021-09-10 |
 PREQUEL 88813638 not registered Live/Pending |
EPC IP Co. LLC 2020-02-27 |
 PREQUEL 88190978 not registered Live/Pending |
Myriad Genetics, Inc. 2018-11-12 |
 PREQUEL 88153103 5762396 Live/Registered |
PREQUEL INC. 2018-10-12 |
 PREQUEL 87821653 not registered Live/Pending |
CapTech Ventures, Inc. 2018-03-06 |
 PREQUEL 87590660 not registered Dead/Abandoned |
SINO LEGACY HOLDINGS LIMITED 2017-08-30 |
 PREQUEL 87224831 5325519 Live/Registered |
Jackson Family Farms, LLC 2016-11-03 |
 PREQUEL 86980332 5075234 Live/Registered |
Epoch Lacrosse LLC 2015-08-03 |
 PREQUEL 86713121 not registered Dead/Abandoned |
Epoch Lacrosse LLC 2015-08-03 |
 PREQUEL 86022257 not registered Dead/Abandoned |
PL360 Beverage Partners, LLC 2013-07-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.