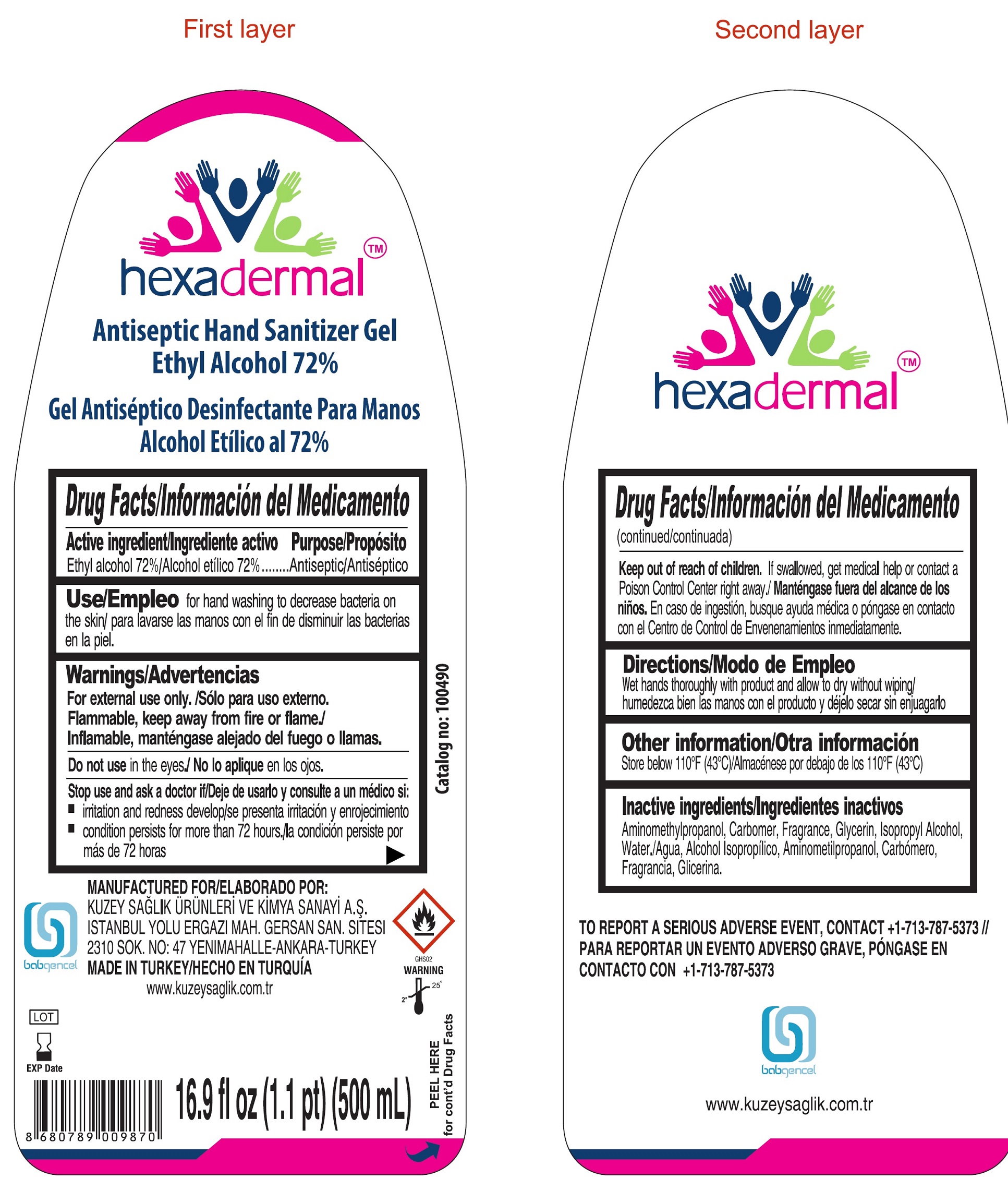
HEXADERMAL ANTISEPTIC HAND SANITIZER
HEXADERMAL ANTISEPTIC HAND SANITIZER by
Drug Labeling and Warnings
HEXADERMAL ANTISEPTIC HAND SANITIZER by is a Otc medication manufactured, distributed, or labeled by KUZEY SAGLIK URUNLERI VE KIMYA SANAYI ANONIM SIRKETI KAZAN SUBESI. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HEXADERMAL ANTISEPTIC HAND SANITIZER- alcohol gel
KUZEY SAGLIK URUNLERI VE KIMYA SANAYI ANONIM SIRKETI KAZAN SUBESI
----------
HEXADERMAL ANTISEPTIC HAND SANITIZER
| HEXADERMAL ANTISEPTIC HAND SANITIZER
alcohol gel |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - KUZEY SAGLIK URUNLERI VE KIMYA SANAYI ANONIM SIRKETI KAZAN SUBESI (519910295) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| KUZEY SAGLIK URUNLERI VE KIMYA SANAYI ANONIM SIRKETI KAZAN SUBESI | 519910295 | manufacture(80256-000) | |
Revised: 9/2024
Document Id: 21752509-8cb0-5157-e063-6394a90a5f1f
Set id: ffd9ead9-2710-4c1b-87ba-261a6fded1be
Version: 4
Effective Time: 20240906
KUZEY SAGLIK URUNLERI VE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.