CyFolex by Key Therapeutics, LLC Cyfolex
CyFolex by
Drug Labeling and Warnings
CyFolex by is a Other medication manufactured, distributed, or labeled by Key Therapeutics, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
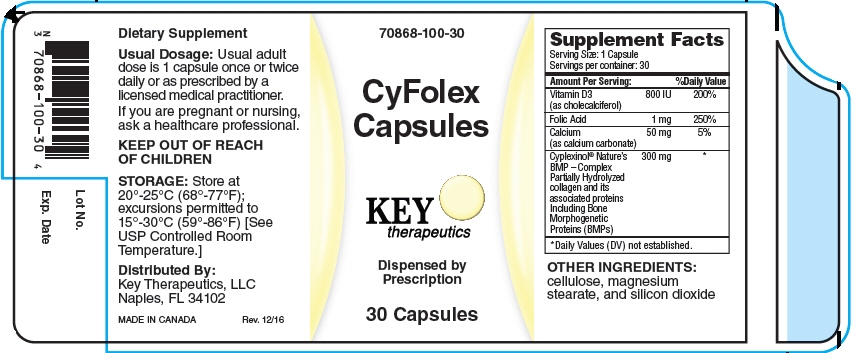
CYFOLEX- cholecalciferol, folic acid, calcium carbonate, and bovine type i collagen capsule
Key Therapeutics
----------
Cyfolex
Supplement Facts
| Serving Size: 1 Capsule |
Servings per container: 30 |
|
| Amount Per Serving: | % Daily Value | |
| Vitamin D3 (as cholecalciferol) | 800 IU | 200% |
| Folic Acid | 1 mg | 250% |
| Calcium (as calcium carbonate) | 50 mg | 5% |
| Cyplexinol® Nature's BMP – a Complex Partially Hydrolyzed collagen and its associated proteins Including Bone Morphogenetic Proteins (BMPs) |
300 mg |
* |
* Daily Values (DV) not established.
OTHER INGREDIENTS
cellulose, magnesium stearate, and silicon dioxide.
DESCRIPTION
CyFolex Capsules is an orally administered prescription folic acid supplement for the clinical dietary management of suboptimal nutritional status in patients where advanced folate supplementation is required, and nutritional supplementation for maintenance of good bone and cartilage health is needed.
CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNINGS
PRECAUTIONS
CyFolex Capsules should only be used under the direction and supervision of a licensed medical practitioner.
Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress.
Pregnancy and Lactation
CyFolex Capsules is not intended for use in pregnant or lactating patients.
ADVERSE REACTIONS
You should call your doctor for medical advice about serious adverse events. To report adverse side effects or to obtain product information, contact Key Therapeutics, LLC, Naples FL 34102 at 1-888- 981-8337.
DOSAGE AND ADMINISTRATION
Usual adult dose is 1 capsule once or twice daily or as prescribed by a licensed medical practitioner.
HOW SUPPLIED
CyFolex Capsules are supplied as light yellow capsules, printed with "100" dispensed in a child- resistant bottle of 30ct. (70868-100-301)
1 Key Therapeutics, LLC does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply- chain control including pharmacies.
STORAGE
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature.]
Protect from heat, light and moisture.
KEEP OUT OF THE REACH OF CHILDREN.
Tamper Evident
Do not use if seal is broken or missing.
Manufactured for:
Key Therapeutics, LLC Naples, FL 34102 MADE IN CANADA
Dispensed by Prescription. Rev. 12/16
†This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760). The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription (Rx). This is not an Orange Book product. This product may be administered only under a physician's supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
| CYFOLEX
cholecalciferol, folic acid, calcium carbonate, and bovine type i collagen capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Supplement Facts | ||
| Serving Size : | Serving per Container : | |
| Amount Per Serving | % Daily Value | |
|---|---|---|
| color | ||
| imprint | ||
| scoring | 1 | |
| shape | ||
| size (solid drugs) | 15 mm | |
| Labeler - Key Therapeutics (080318791) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.