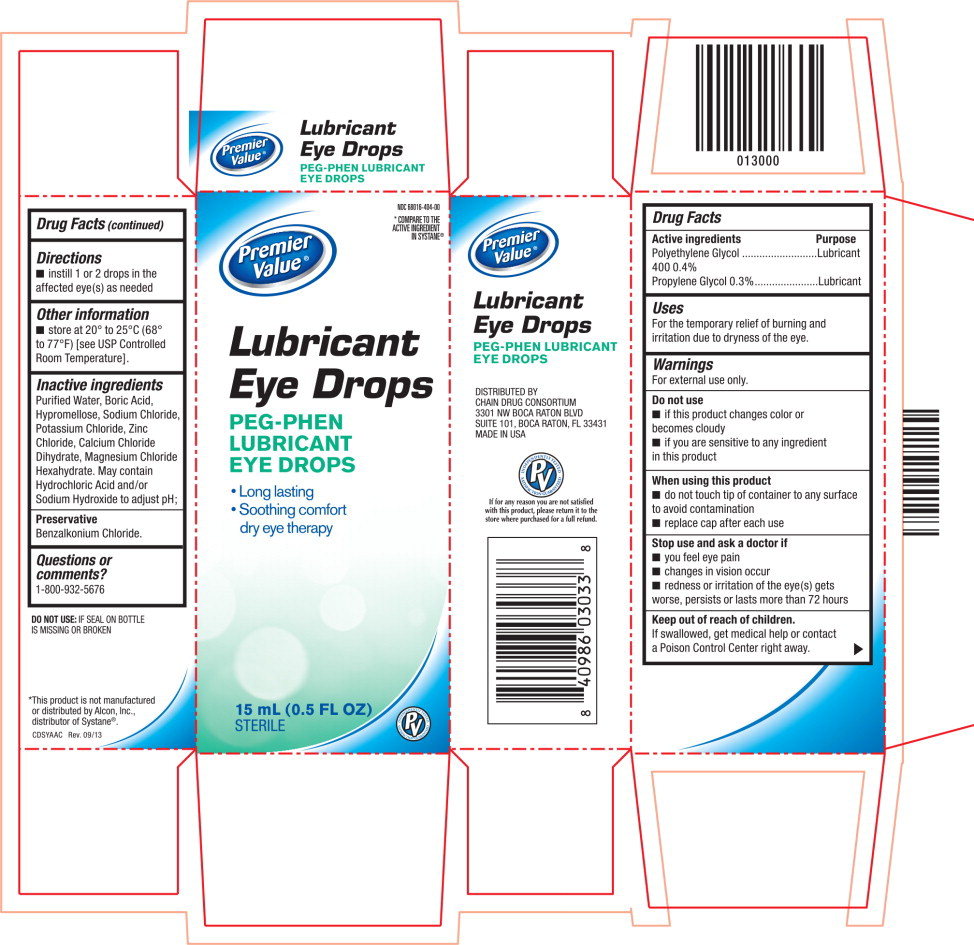
LUBRICANT EYE DROPS- polyethylene glycol 400 and propylene glycol solution/ drops
Lubricant Eye Drops by
Drug Labeling and Warnings
Lubricant Eye Drops by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, Akorn, Inc. , Akorn, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only.
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LUBRICANT EYE DROPS
polyethylene glycol 400 and propylene glycol solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68016-404 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Polyethylene Glycol 400 (UNII: B697894SGQ) (Polyethylene Glycol 400 - UNII:B697894SGQ) Polyethylene Glycol 400 4 mg in 1 mL Propylene Glycol (UNII: 6DC9Q167V3) (Propylene Glycol - UNII:6DC9Q167V3) Propylene Glycol 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Boric Acid (UNII: R57ZHV85D4) Hypromelloses (UNII: 3NXW29V3WO) Sodium Chloride (UNII: 451W47IQ8X) Potassium Chloride (UNII: 660YQ98I10) Zinc Chloride (UNII: 86Q357L16B) Calcium Chloride (UNII: M4I0D6VV5M) Magnesium Chloride (UNII: 02F3473H9O) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Benzalkonium Chloride (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68016-404-00 1 in 1 CARTON 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 01/10/2014 Labeler - Chain Drug Consortium (101668460) Registrant - Akorn, Inc. (062649876) Establishment Name Address ID/FEI Business Operations Akorn, Inc 603980319 MANUFACTURE(68016-404) , ANALYSIS(68016-404) , STERILIZE(68016-404) , PACK(68016-404) , LABEL(68016-404)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.