Oxyband
GUDID 00009235421772
Oxyband 4.5 X 5.5 Dressing
The Lighthouse For The Blind
Wound therapy oxygenator dressing| Primary Device ID | 00009235421772 |
| NIH Device Record Key | b78f53d6-2453-4fb1-b2d0-4bc49a49bb13 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Oxyband |
| Version Model Number | 1 |
| Company DUNS | 051731214 |
| Company Name | The Lighthouse For The Blind |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com | |
| Phone | 877.774.4211 |
| OxybandUS@semlerscientific.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00009235421772 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K043063
FDA Product Code
| FRO | Dressing, Wound, Drug |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-10-13 |
| Device Publish Date | 2021-10-05 |
On-Brand Devices [Oxyband]
| 00009235421772 | Oxyband 4.5 X 5.5 Dressing |
| 00009235000007 | Oxyband 7 X 9 Dressing |
Trademark Results [Oxyband]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
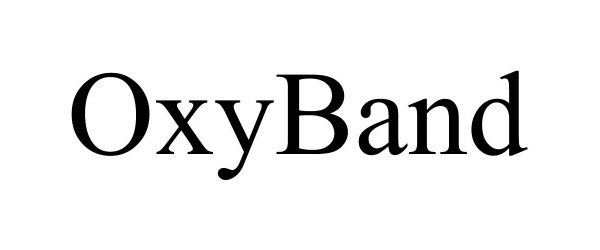 OXYBAND 90276680 not registered Live/Pending |
Oxyband Technologies, Inc 2020-10-25 |
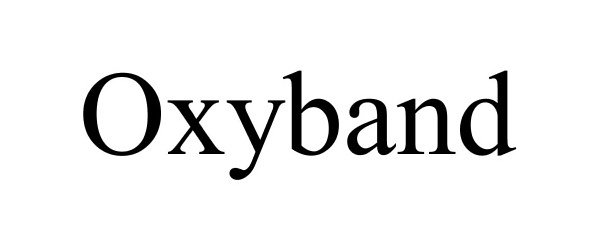 OXYBAND 87409241 not registered Dead/Abandoned |
OXYBAND TECHNOLOGIES, INC. 2017-04-12 |
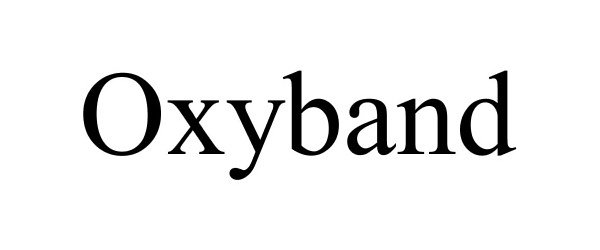 OXYBAND 87069970 not registered Dead/Abandoned |
OXYBAND TECHNOLOGIES, INC. 2016-06-13 |
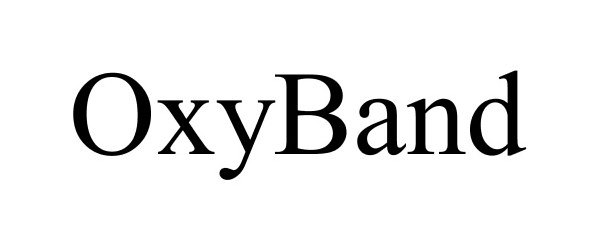 OXYBAND 77964013 not registered Dead/Abandoned |
OxyBand Technologies Inc. 2010-03-19 |
 OXYBAND 76534706 3145872 Dead/Cancelled |
Hansen, Conrad J. 2003-07-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.