TRUEcontrol
GUDID 00021292002107
TRUEcontrol Solution Level 1
TRIVIDIA HEALTH, INC.
Glucose IVD, control| Primary Device ID | 00021292002107 |
| NIH Device Record Key | d60378e0-8986-47a1-80cc-cc0369c63cf2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TRUEcontrol |
| Version Model Number | TRUEcontrol Solution Level 1 |
| Company DUNS | 151810868 |
| Company Name | TRIVIDIA HEALTH, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
Operating and Storage Conditions
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
| Handling Environment Temperature | Between 36 Degrees Fahrenheit and 86 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *DO NOT REFRIGERATE OR FREEZE |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00021292002107 [Package] Contains: 00021292008369 Package: box [1 Units] In Commercial Distribution |
| GS1 | 00021292008369 [Primary] |
| GS1 | 10021292002104 [Package] Package: CASE [400 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K032657
FDA Product Code
| JJX | Single (Specified) Analyte Controls (Assayed And Unassayed) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-02-08 |
| Device Publish Date | 2021-01-29 |
On-Brand Devices [TRUEcontrol]
| 00021292002091 | TRUEcontrol Solution Level 0 |
| 00021292008376 | TRUEcontrol Solution Level 2 |
| 00021292002107 | TRUEcontrol Solution Level 1 |
Trademark Results [TRUEcontrol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRUECONTROL 88644691 not registered Live/Pending |
RHA Technologies Ltd 2019-10-07 |
 TRUECONTROL 88045085 not registered Dead/Abandoned |
Mediashift Technologies, Inc. 2018-07-19 |
 TRUECONTROL 86170835 4683768 Live/Registered |
TRIVIDIA HEALTH, INC. 2014-01-21 |
 TRUECONTROL 85229781 not registered Dead/Abandoned |
Savant Systems LLC 2011-01-31 |
 TRUECONTROL 78520242 3041351 Dead/Cancelled |
Rendition Networks, Inc. 2004-11-19 |
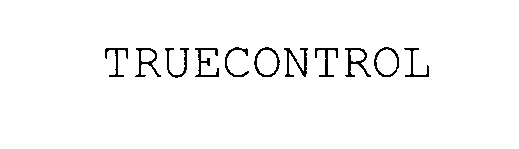 TRUECONTROL 78025355 not registered Dead/Abandoned |
Abbott Laboratories 2000-09-11 |
 TRUECONTROL 77365927 3515317 Dead/Cancelled |
NIPRO DIAGNOSTICS, INC. 2008-01-07 |
 TRUECONTROL 77172892 3368510 Dead/Cancelled |
NIPRO DIAGNOSTICS, INC. 2007-05-04 |
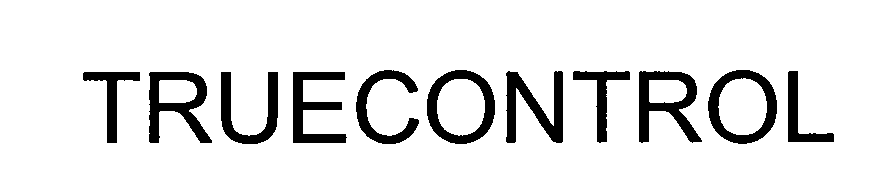 TRUECONTROL 76481894 not registered Dead/Abandoned |
Rendition Networks, Inc. 2003-01-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.