Inject-Ease IE400
GUDID 00025294874007
Holder for disposable syringes that hides the needle and makes self-injections easier
AMBI, INC.
Syringe-loaded medication/vaccine injector, manual, professional| Primary Device ID | 00025294874007 |
| NIH Device Record Key | 040a0e25-6411-4b14-94ed-c7f086937954 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Inject-Ease |
| Version Model Number | IE400 |
| Catalog Number | IE400 |
| Company DUNS | 929744212 |
| Company Name | AMBI, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 831-475-1765 |
| info@ambimedinc.com | |
| Phone | 831-475-1765 |
| info@ambimedinc.com | |
| Phone | 831-475-1765 |
| info@ambimedinc.com | |
| Phone | 831-475-1765 |
| info@ambimedinc.com | |
| Phone | 831-475-1765 |
| info@ambimedinc.com | |
| Phone | 831-475-1765 |
| info@ambimedinc.com | |
| Phone | 831-475-1765 |
| info@ambimedinc.com |
Device Dimensions
| Length | 5.4 Inch |
| Width | 1.7 Inch |
| Length | 5.4 Inch |
| Width | 1.7 Inch |
| Length | 5.4 Inch |
| Width | 1.7 Inch |
| Length | 5.4 Inch |
| Width | 1.7 Inch |
| Length | 5.4 Inch |
| Width | 1.7 Inch |
| Length | 5.4 Inch |
| Width | 1.7 Inch |
| Length | 5.4 Inch |
| Width | 1.7 Inch |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00025294874007 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K872233
FDA Product Code
| KZH | Introducer, Syringe Needle |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2022-06-10 |
| Device Publish Date | 2018-12-17 |
Trademark Results [Inject-Ease]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
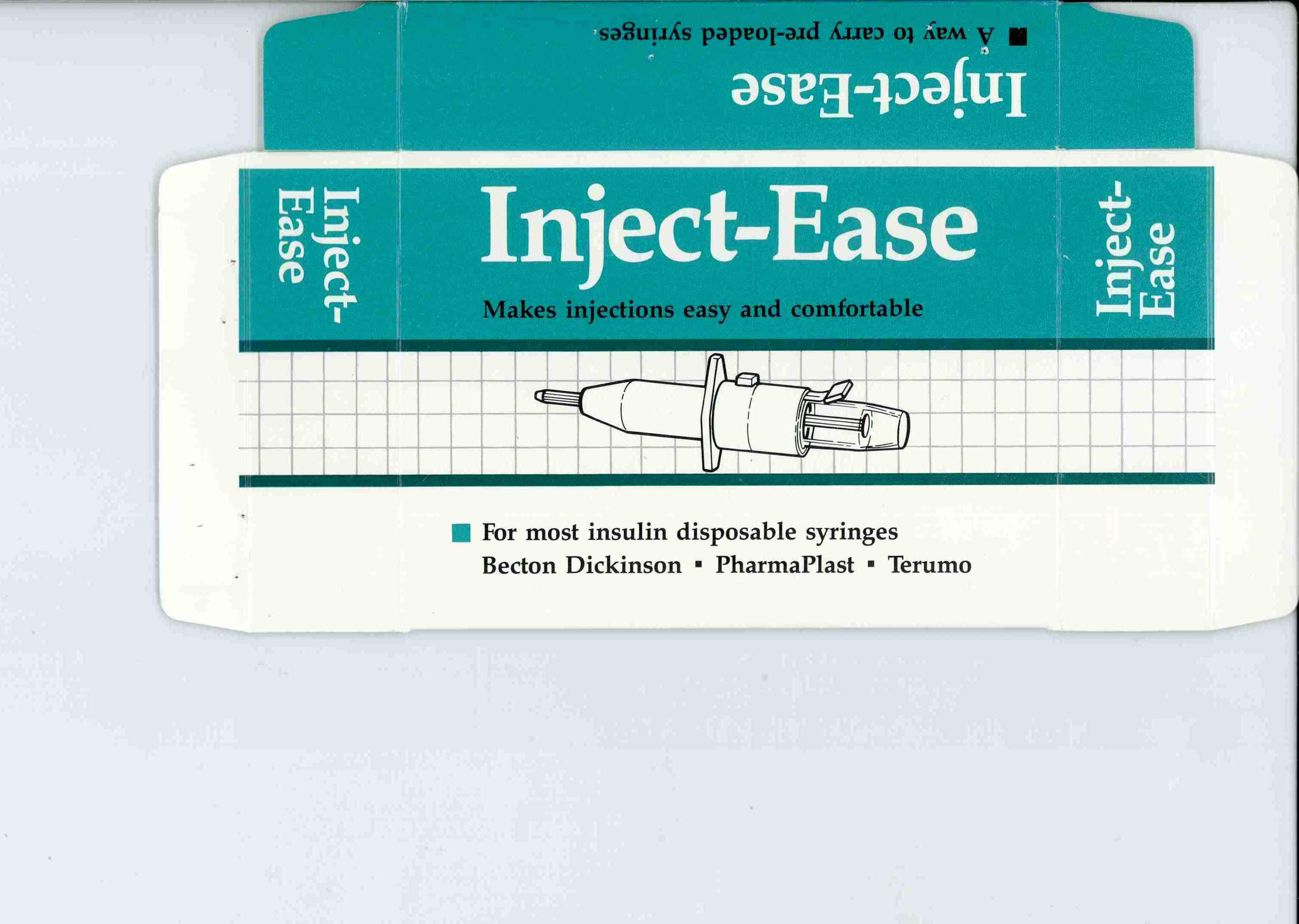 INJECT-EASE 73770310 1557817 Live/Registered |
LEVIN, PAUL D. 1988-12-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.