Kidgets
GUDID 00032251574399
Sunglasses (non-prescription) Regulation Number 886.5850 Device Class 1
Family Dollar Stores
Tinted spectacles, non-prescription| Primary Device ID | 00032251574399 |
| NIH Device Record Key | ad6f097d-7eb9-4646-a518-23a5fb2240f1 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Kidgets |
| Version Model Number | SKU#1699231 |
| Company DUNS | 117310689 |
| Company Name | Family Dollar Stores |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com | |
| Phone | 212-391-6970 |
| elee@shalomint.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00032251574399 [Primary] |
FDA Product Code
| HQY | Sunglasses (Non-Prescription Including Photosensitive) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-05-11 |
| Device Publish Date | 2020-05-01 |
Devices Manufactured by Family Dollar Stores
| 20032251689653 - FAMILY WELLNESS | 2025-08-11 FW FIRST SIGNAL PREG TEST 2CT |
| 10032251689663 - FAMILY WELLNESS | 2025-08-11 FW FIRST SIGNAL PREG TEST 1CT |
| 10032251689885 - FAMILY WELLNESS | 2025-08-11 FW FIRST SIGNAL PREG TEST CARTRIDGE 1CT |
| 20032251911808 - Family Wellness | 2024-07-16 COMPACT TAMPON REG |
| 20032251911792 - Family Wellness | 2024-07-16 COMPACT TAMPON SUPER |
| 20032251911846 - Family Wellness | 2024-07-16 PLASTIC TAMPON SUPER |
| 20032251911853 - Family Wellness | 2024-07-16 PLASTIC TAMPON SUPER |
| 20032251911860 - Family Wellness | 2024-07-16 PLASTIC TAMPON REGULAR |
Trademark Results [Kidgets]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
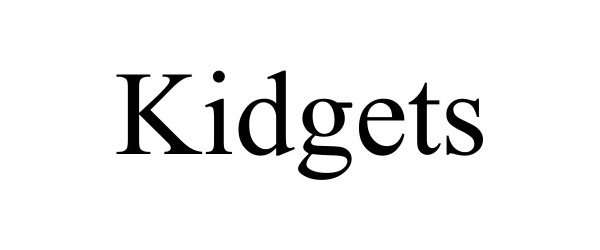 KIDGETS 98360763 not registered Live/Pending |
TinyByte Apps LLC 2024-01-17 |
 KIDGETS 77983004 4187209 Live/Registered |
FAMILY DOLLAR IP CO., LLC 2009-05-29 |
 KIDGETS 77982092 4013303 Live/Registered |
FAMILY DOLLAR IP CO., LLC 2009-05-29 |
 KIDGETS 77981202 3932627 Live/Registered |
FAMILY DOLLAR IP CO., LLC 2009-05-29 |
 KIDGETS 77919048 not registered Dead/Abandoned |
Family Dollar Stores of Michigan, Inc. 2010-01-25 |
 KIDGETS 77764237 3813046 Live/Registered |
FAMILY DOLLAR IP CO., LLC 2009-06-19 |
 KIDGETS 77748002 4301629 Live/Registered |
FAMILY DOLLAR IP CO., LLC 2009-05-29 |
 KIDGETS 75527118 2464212 Live/Registered |
FAMILY DOLLAR IP CO., LLC 1998-07-27 |
 KIDGETS 74137666 1738404 Dead/Cancelled |
FAMILY DOLLAR SERVICES, INC. 1991-02-08 |
 KIDGETS 73634126 1450943 Dead/Cancelled |
FAMILY DOLLAR STORES, INC. 1986-12-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.