Poise
GUDID 00036000411188
POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE
KIMBERLY-CLARK GLOBAL SALES, INC.
Urinary-incontinence vaginal insert, single-use| Primary Device ID | 00036000411188 |
| NIH Device Record Key | 81d3c558-c8d3-49d7-9f1f-502f7ffeade1 |
| Commercial Distribution Discontinuation | 2019-04-04 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Poise |
| Version Model Number | Pessary |
| Company DUNS | 829568273 |
| Company Name | KIMBERLY-CLARK GLOBAL SALES, INC. |
| Device Count | 10 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx | |
| Phone | +1(800)340-3102 |
| xxx@xxx.xxx |
Device Dimensions
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
| Width | 50 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00036000411188 [Primary] |
| GS1 | 00036000998467 [Unit of Use] |
| GS1 | 10036000411185 [Package] Package: Case [4 Units] Discontinued: 2019-04-04 Not in Commercial Distribution |
FDA Product Code
| HHW | PESSARY, VAGINAL |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-04-04 |
| Device Publish Date | 2017-03-14 |
On-Brand Devices [Poise]
| 00036000411188 | POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE |
| 00036000411171 | POISE IMPRESSA SIZE 3 BLADDER SUPPORT DEVICE |
| 10036000411130 | POISE IMPRESSA SIZE 1 BLADDER SUPPORT DEVICE |
| 00036000496239 | POISE IMPRESSA SIZE 1 BLADDER SUPPORT DEVICE |
| 00036000496222 | POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE |
| 00036000496215 | POISE IMPRESSA SIZE 3 BLADDER SUPPORT DEVICE |
| 00036000496208 | POISE IMPRESSA SIZE 3 BLADDER SUPPORT DEVICE |
| 00036000496192 | POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE |
| 00036000496185 | POISE IMPRESSA SIZE 1 BLADDER SUPPORT DEVICE |
| 00036000470611 | POISE IMPRESSA SIZE 3 BLADDER SUPPORT DEVICE |
| 00036000470604 | POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE |
| 00036000470598 | POISE IMPRESSA SIZE 1 BLADDER SUPPORT DEVICE |
| 10036000465607 | POISE IMPRESSA SIZE 3 BLADDER SUPPORT DEVICE |
| 10036000465591 | POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE |
| 10036000465584 | POISE IMPRESSA SIZE 1 BLADDER SUPPORT DEVICE |
| 00036000431933 | POISE IMPRESSA SIZE 3 BLADDER SUPPORT DEVICE |
| 00036000431926 | POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE |
| 00036000431919 | POISE IMPRESSA SIZE 1 BLADDER SUPPORT DEVICE |
| 10036000411161 | POISE IMPRESSA BLADDER SUPPORT DEVICE |
| 00036000471335 | POISE IMPRESSA MIXED BLADDER SUPPORT DEVICE 2.541-CASE DISPLAY 13-PKGS |
| 00036000471328 | POISE IMPRESSA MIXED BLADDER SUPPORT DEVICE 2.5-CASE DISPLAY 10-PACKAGES |
| 00036000470963 | POISE IMPRESSA MIXED BLADDER SUPPORT DEVICE 2-CASE DISPLAY 8-PACKAGES |
| 00036000462111 | POISE IMPRESSA BLADDER SUPPORT DEVICE MIXED 3-CS DISPLAY 12-PKG |
| 00036000462074 | POISE IMPRESSA BLADDER SUPPORT DEVICE MIXED 4-CS DIS 16 PKG |
| 00036000411164 | POISE IMPRESSA BLADDER SUPPORT DEVICE |
| 00036000536829 | ONE BY POISE SUPRPREM ULTRATHN REGWNG PAD E-COMM 66 |
| 00036000536836 | ONE BY POISE SUPRPREM XCVRG FLAT LINER E-COMM 150 |
| 10036000534501 | ONE BY POISE SUPRPREM ULTRATHN HVYWNG PAD 18 |
| 10036000534495 | ONE BY POISE SUPRPREM ULTRATHN REGWNG PAD 22 |
| 10036000534488 | ONE BY POISE SUPRPREM XCVRG FLAT LINER 50 |
| 00036000670011 | Sample pack containing one of each: 1) One by Poise Extra Coverage Liner,2) One by Poise Heavy P |
| 00036000541953 | ONE BY POISE SUPRPREM ULTRATHN HVYWNG PAD MIX DSP |
| 00036000541786 | ONE BY POISE PAD MIX DSP |
| 00036000597219 | Sample pack contains 2 liners of: One by Poise Extra Coverage Liners, 1 Cottonelle Flushable Wip |
| 00036000670035 | Sample pack contains one of each: 1) One by Poise Extra Coverage Liner (53451-00),2) One by Pois |
| 10036000491378 | POISE IMPRESSA SIZING KIT BLADDER SUPPORT DEVICE 3 |
| 10036000470625 | POISE IMPRESSA SIZING KIT BLADDER SUPPORT DEVICE 6 |
| 10036000470618 | POISE IMPRESSA SIZE 3 BLADDER SUPPORT DEVICE |
| 10036000470601 | POISE IMPRESSA SIZE 2 BLADDER SUPPORT DEVICE |
| 10036000470595 | POISE IMPRESSA SIZE 1 BLADDER SUPPORT DEVICE |
| 10036000465690 | POISE IMPRESSA BLADDER SUPPORT DEVICE |
| 00036000491371 | POISE IMPRESSA SIZING KIT BLADDER SUPPORT DEVICE 3 |
| 00036000475753 | POISE IMPRESSA MIXED BLADDER SUPPORT DEVICE 1-CASE DISPLAY 4-PACKAGES |
| 00036000470628 | POISE IMPRESSA SIZING KIT BLADDER SUPPORT DEVICE 6 |
| 00036000465693 | POISE IMPRESSA BLADDER SUPPORT DEVICE |
Trademark Results [Poise]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 POISE 98054536 not registered Live/Pending |
Kimberly-Clark Worldwide, Inc. 2023-06-22 |
 POISE 88368996 not registered Live/Pending |
Johnson & Johnson 2019-04-03 |
 POISE 87722712 5519286 Live/Registered |
Fantastic Industries, Inc. 2017-12-15 |
 POISE 87573409 not registered Dead/Abandoned |
Helena Holding Company 2017-08-17 |
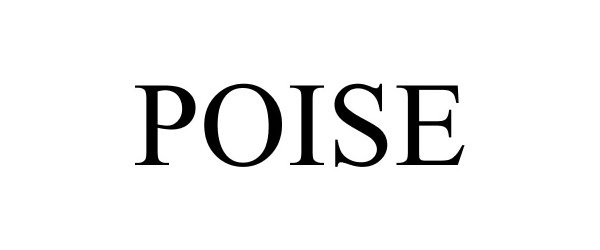 POISE 87119143 not registered Dead/Abandoned |
ROYAL WINE CORPORATION 2016-07-28 |
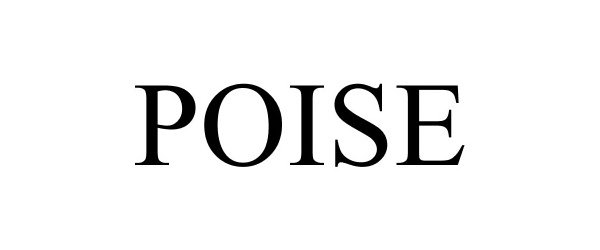 POISE 86827394 5094014 Live/Registered |
Marquis, David C. 2015-11-20 |
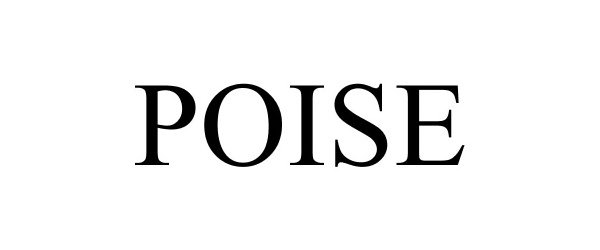 POISE 86315135 not registered Dead/Abandoned |
Marquis, David C. 2014-06-19 |
 POISE 86236719 4613915 Live/Registered |
KIMBERLY-CLARK WORLDWIDE, INC. 2014-03-31 |
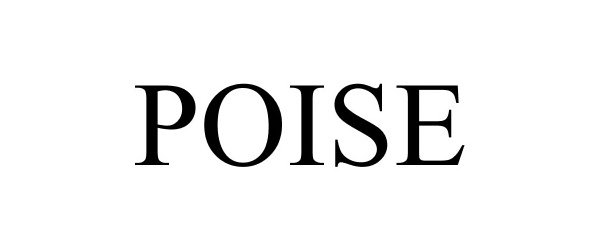 POISE 85956587 4637975 Live/Registered |
Kimberly-Clark Worldwide, Inc. 2013-06-11 |
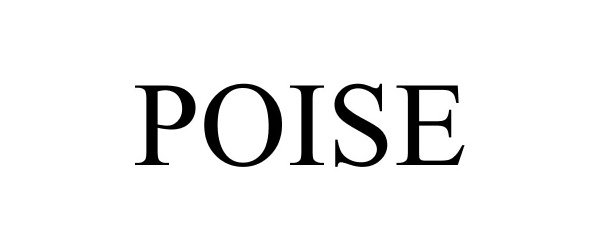 POISE 85709054 4280015 Dead/Cancelled |
KIMBERLY-CLARK WORLDWIDE, INC. 2012-08-21 |
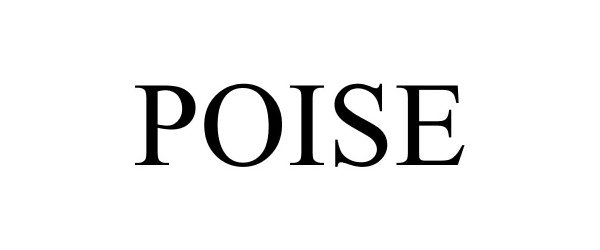 POISE 85688416 4279971 Dead/Cancelled |
KIMBERLY-CLARK WORLDWIDE, INC. 2012-07-27 |
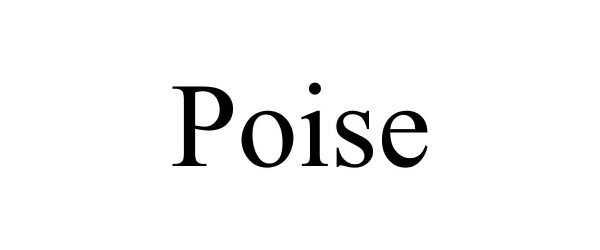 POISE 85651288 not registered Dead/Abandoned |
Poison LLC 2012-06-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.