Being Well
GUDID 00051933656895
Being Well Home Pregnancy Test
Save-Mor Beauty & Barber Supply, Inc.
Total human chorionic gonadotropin IVD, kit, rapid ICT, clinical| Primary Device ID | 00051933656895 |
| NIH Device Record Key | b815c879-535c-470e-b4c7-876043537d5a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Being Well |
| Version Model Number | 65689 |
| Company DUNS | 052149069 |
| Company Name | Save-Mor Beauty & Barber Supply, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00051933656895 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K150022
FDA Product Code
| LCX | Kit, Test, Pregnancy, Hcg, Over The Counter |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2026-02-04 |
| Device Publish Date | 2026-01-27 |
Trademark Results [Being Well]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
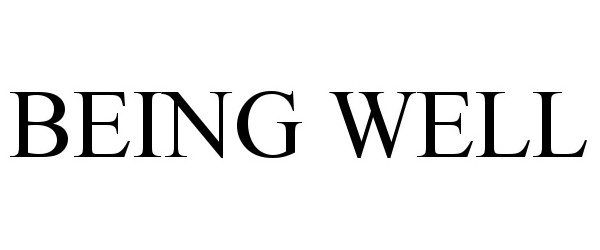 BEING WELL 98015798 not registered Live/Pending |
Moran Foods, LLC 2023-05-26 |
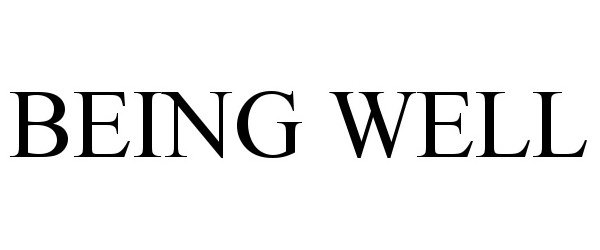 BEING WELL 97330086 not registered Live/Pending |
Moran Foods, LLC 2022-03-25 |
 BEING WELL 87766787 not registered Live/Pending |
Live Wellness, Inc. 2018-01-23 |
 BEING WELL 76621853 3114739 Live/Registered |
MORAN FOODS, LLC 2004-11-23 |
 BEING WELL 74448932 1862484 Dead/Cancelled |
QualMed, Inc. 1993-10-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.