TARGET
GUDID 00080196285955
UP WRAP ELASTIC BANDAGE
TARGET CORPORATION
Ankle binder, single-use| Primary Device ID | 00080196285955 |
| NIH Device Record Key | fedd1ca4-dba7-4904-8adf-161ac2d770d5 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TARGET |
| Version Model Number | TGT0426B |
| Company DUNS | 006961700 |
| Company Name | TARGET CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com | |
| Phone | 847-643-3859 |
| vallen@medline.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00080196285955 [Primary] |
FDA Product Code
| FQM | Bandage, Elastic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-09-25 |
| Device Publish Date | 2020-09-17 |
On-Brand Devices [TARGET]
| 00080196285955 | UP WRAP ELASTIC BANDAGE |
| 00888277716325 | Pink Pre-Cut Kinesiology Tape - 2in x 10in - Up&Up |
| 00888277716318 | Black Pre-Cut Kinesiology Tape - 2in x 10in - Up&Up |
Trademark Results [TARGET]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
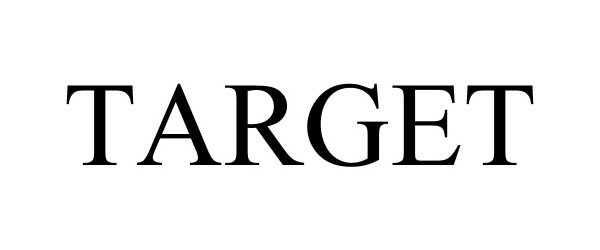 TARGET 98202538 not registered Live/Pending |
TARGET GESTÃO DE MARCAS E PATENTES LTDA 2023-09-28 |
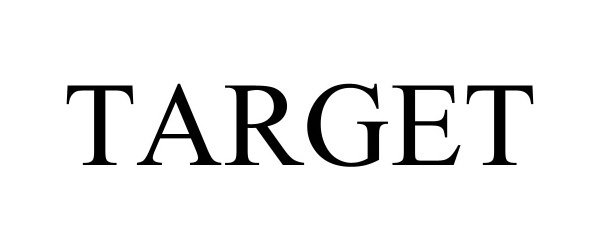 TARGET 98073630 not registered Live/Pending |
TERELION, LLC 2023-07-06 |
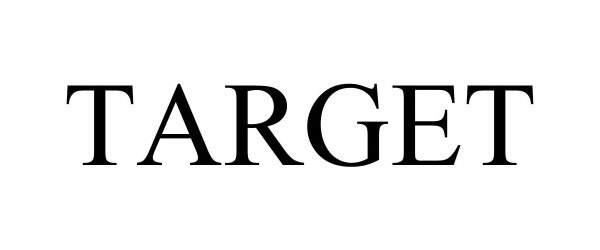 TARGET 97931822 not registered Live/Pending |
Target Brands, Inc. 2023-05-11 |
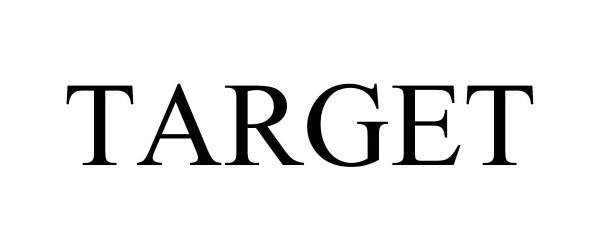 TARGET 97368207 not registered Live/Pending |
Target Brands, Inc. 2022-04-18 |
 TARGET 97185019 not registered Live/Pending |
Target Brands, Inc. 2021-12-22 |
 TARGET 90391060 not registered Live/Pending |
Quikrete International, Inc. 2020-12-17 |
 TARGET 90345833 not registered Live/Pending |
Target Brands, Inc. 2020-11-27 |
 TARGET 88126991 not registered Live/Pending |
KAPS, THERESA 2018-09-21 |
 TARGET 88126991 not registered Live/Pending |
KAPS, THOMAS 2018-09-21 |
 TARGET 88126991 not registered Live/Pending |
ROSARIO, JEANNE 2018-09-21 |
 TARGET 88126991 not registered Live/Pending |
ROSARIO, PAUL 2018-09-21 |
 TARGET 87850818 not registered Live/Pending |
Target Brands, Inc. 2018-03-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.