Pro Lift
GUDID 00190837021610
Life Spine, Inc.
Metallic spinal fusion cage, non-sterile| Primary Device ID | 00190837021610 |
| NIH Device Record Key | 1c3683b2-c1c8-4fac-b78b-8d14820a6a39 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Pro Lift |
| Version Model Number | 58-1228-0708P |
| Company DUNS | 183641617 |
| Company Name | Life Spine, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00190837021610 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K173182
FDA Product Code
| MAX | Intervertebral Fusion Device With Bone Graft, Lumbar |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2018-09-03 |
| Device Publish Date | 2018-08-01 |
On-Brand Devices [Pro Lift]
| 00190837021610 | 58-1228-0708P |
| 00190837021535 | 58-1228-0710P |
| 00190837021504 | 58-1228-0813P |
| 00190837030940 | 151-261 |
| 00190837091033 | 2019-0080 |
| 00190837091026 | 129-1018 |
| 00190837091019 | 129-1017 |
Trademark Results [Pro Lift]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
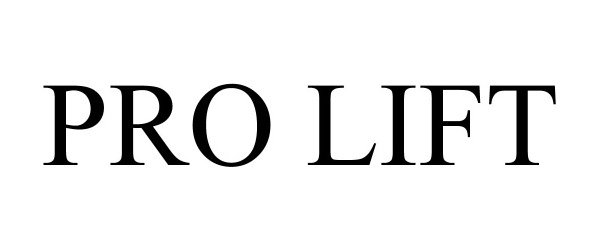 PRO LIFT 97404686 not registered Live/Pending |
Nielsen Products, LLC 2022-05-11 |
 PRO LIFT 86190353 4620199 Live/Registered |
Nielsen Products, LLC 2014-02-11 |
 PRO LIFT 85824793 not registered Dead/Abandoned |
Nielsen Products, LLC 2013-01-16 |
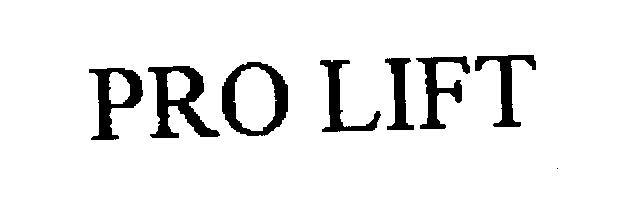 PRO LIFT 76266174 2644287 Dead/Cancelled |
Loren S. Fond, Inc. 2001-06-01 |
 PRO LIFT 75596156 not registered Dead/Abandoned |
HUFFY CORPORATION 1998-11-19 |
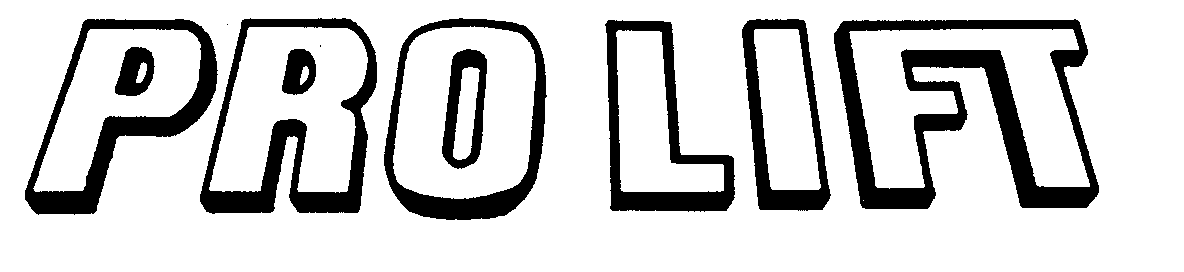 PRO LIFT 73506693 1340578 Dead/Cancelled |
ANDERSON, HAROLD R. 1984-11-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.