AlloWedge 55-E-22-22-12
GUDID 00191083047379
EVANS TRIAL ASSEMBLY, 12mm
Rti Surgical, Inc.
Surgical implant/trial-implant/sizer holder, reusable| Primary Device ID | 00191083047379 |
| NIH Device Record Key | 343860df-b5b8-4821-8bcd-18735641c004 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | AlloWedge |
| Version Model Number | 55-E-22-22-12 |
| Catalog Number | 55-E-22-22-12 |
| Company DUNS | 117560455 |
| Company Name | Rti Surgical, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com | |
| Phone | +1(386)418-8888 |
| labeling@rtix.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00191083047379 [Primary] |
FDA Product Code
| LHX | Trousers, anti-shock |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
[00191083047379]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-10-22 |
| Device Publish Date | 2024-10-14 |
On-Brand Devices [AlloWedge]
| 00191083047416 | Sterilization Case |
| 00191083047409 | Distractor, Narrow, Foot and Ankle Wedge |
| 00191083047393 | Distractor, Foot and Ankle Wedge |
| 00191083047386 | Mallet - Foot and Ankle Wedge |
| 00191083047379 | EVANS TRIAL ASSEMBLY, 12mm |
| 00191083047362 | EVANS TRIAL ASSEMBLY, 10mm |
| 00191083047355 | EVANS TRIAL ASSEMBLY, 8mm |
| 00191083047348 | EVANS TRIAL ASSEMBLY, 6mm |
| 00191083047331 | COTTON TRIAL ASSEMBLY, 8mm |
| 00191083047324 | COTTON TRIAL ASSEMBLY, 7mm |
| 00191083047317 | COTTON TRIAL ASSEMBLY, 6mm |
| 00191083047300 | COTTON TRIAL ASSEMBLY, 5mm |
| 00191083047294 | OSTEOTOME, FOOT AND ANKLE |
| 00191083047287 | SMALL TAMP, FOOT AND ANKLE |
| 00191083047270 | LARGE TAMP, FOOT AND ANKLE |
Trademark Results [AlloWedge]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
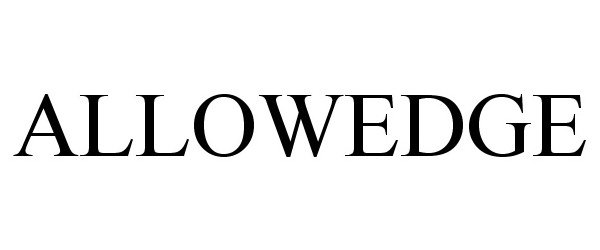 ALLOWEDGE 86303426 4736681 Live/Registered |
RTI Surgical, Inc. 2014-06-08 |
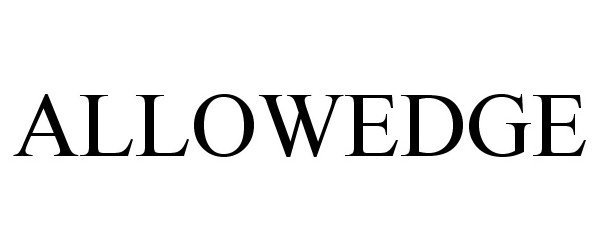 ALLOWEDGE 86303424 4718141 Live/Registered |
RTI Surgical, Inc. 2014-06-08 |
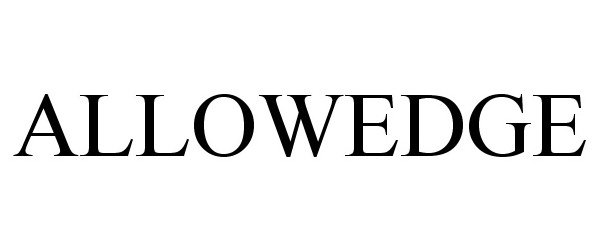 ALLOWEDGE 86302864 4718138 Live/Registered |
RTI Surgical, Inc. 2014-06-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.