StoneSmart™ Connect M006793300D0
GUDID 00191506002527
Console
BOSTON SCIENTIFIC CORPORATION
Flexible video ureteroscope, single-use| Primary Device ID | 00191506002527 |
| NIH Device Record Key | 17d20957-7d31-4a2d-9ff1-63b24ab95208 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | StoneSmart™ Connect |
| Version Model Number | M006793300D0 |
| Catalog Number | M006793300D0 |
| Company DUNS | 021717889 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00191506002527 [Primary] |
FDA Product Code
| FGB | Ureteroscope and accessories, flexible/rigid |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-03-22 |
| Device Publish Date | 2023-03-14 |
On-Brand Devices [StoneSmart™ Connect]
| 00191506002534 | Console |
| 00191506002527 | Console |
| 00191506002510 | Console |
| 00191506002558 | Console |
Trademark Results [StoneSmart]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
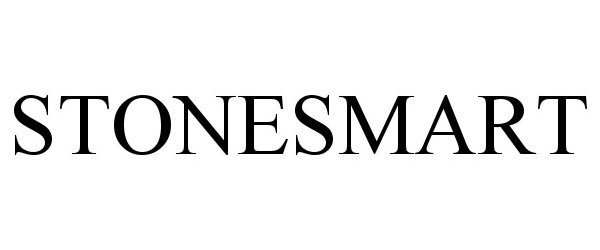 STONESMART 90390225 not registered Live/Pending |
Boston Scientific Scimed, Inc. 2020-12-17 |
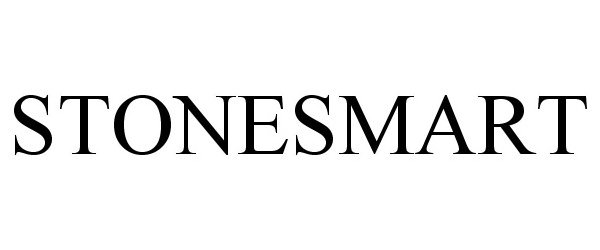 STONESMART 87479015 not registered Live/Pending |
Boston Scientific Scimed, Inc. 2017-06-07 |
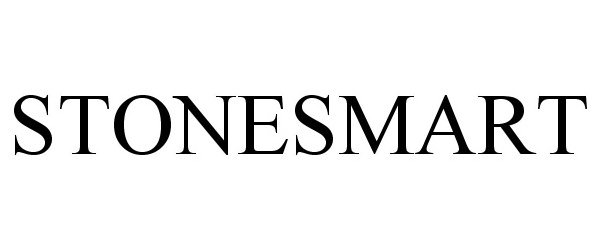 STONESMART 85043610 not registered Dead/Abandoned |
STONEXPRESS, INC. 2010-05-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.