SideKick™ 2
GUDID 00191506013691
Siliconized 10cm x 10mm x 20ga (0.9mm)
BOSTON SCIENTIFIC NEUROMODULATION CORPORATION
Radio-frequency ablation system| Primary Device ID | 00191506013691 |
| NIH Device Record Key | b7ba6a00-f02c-42d3-ad6e-ed666a2f9168 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SideKick™ 2 |
| Version Model Number | RFK-C101020S-ZK2 |
| Company DUNS | 824951958 |
| Company Name | BOSTON SCIENTIFIC NEUROMODULATION CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00191506013103 [Primary] |
| GS1 | 00191506013691 [Package] Contains: 00191506013103 Package: [10 Units] In Commercial Distribution |
FDA Product Code
| GXI | Probe, radiofrequency lesion |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-05-06 |
| Device Publish Date | 2021-04-28 |
On-Brand Devices [SideKick™ 2]
| 00191506013714 | Siliconized 15cm x 10mm x 20ga (0.9mm) |
| 00191506013707 | Siliconized 15cm x 10mm x 18ga (1.2mm) |
| 00191506013691 | Siliconized 10cm x 10mm x 20ga (0.9mm) |
| 00191506013684 | Siliconized 10cm x 10mm x 18ga (1.2mm) |
Trademark Results [SideKick]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SIDEKICK 98762733 not registered Live/Pending |
Mej Consulting LLC 2024-09-21 |
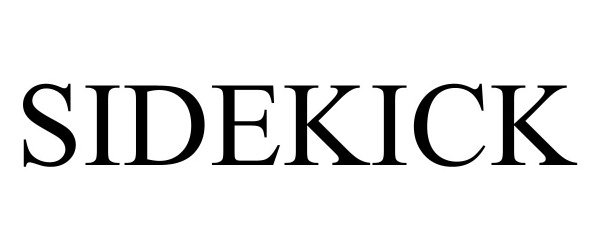 SIDEKICK 98709478 not registered Live/Pending |
SIER TECHNOLOGIES LLC 2024-08-21 |
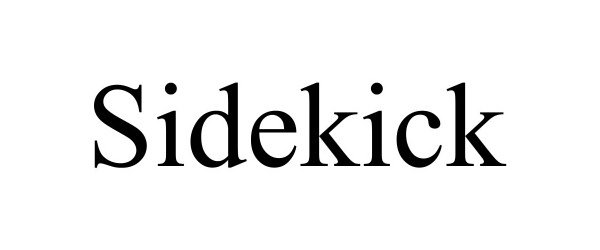 SIDEKICK 98606057 not registered Live/Pending |
WWBD SOLUTIONS INC. 2024-06-18 |
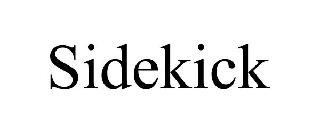 SIDEKICK 98539996 not registered Live/Pending |
AMG LAB LLC 2024-05-08 |
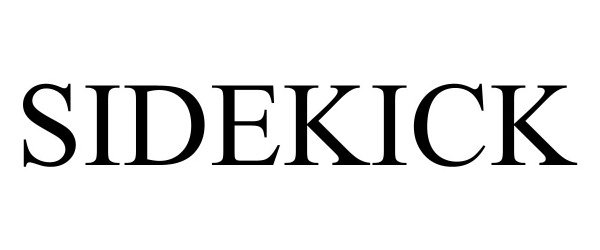 SIDEKICK 98524723 not registered Live/Pending |
Sense It LLC 2024-04-29 |
 SIDEKICK 98349207 not registered Live/Pending |
Sidekick Enterprises, Inc. 2024-01-09 |
 SIDEKICK 98290315 not registered Live/Pending |
Lance Dewbre, 2023-11-29 |
 SIDEKICK 98290315 not registered Live/Pending |
Dewbre, Bill 2023-11-29 |
 SIDEKICK 98142191 not registered Live/Pending |
The Nanny Network, LLC 2023-08-21 |
 SIDEKICK 98141743 not registered Live/Pending |
The Nanny Network, LLC 2023-08-21 |
 SIDEKICK 98141724 not registered Live/Pending |
The Nanny Network, LLC 2023-08-21 |
 SIDEKICK 98027511 not registered Live/Pending |
Dew Beauty Inc. 2023-06-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.