AutoCap™ RX M00545120
GUDID 00191506040215
Integrated Biopsy Cap and Guidewire Locking Device
BOSTON SCIENTIFIC CORPORATION
Endoscopic guidewire holder| Primary Device ID | 00191506040215 |
| NIH Device Record Key | 663b04b0-0650-4c37-9240-7dc5f5629120 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | AutoCap™ RX |
| Version Model Number | M00545120 |
| Catalog Number | M00545120 |
| Company DUNS | 021717889 |
| Company Name | BOSTON SCIENTIFIC CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00191506040208 [Primary] |
| GS1 | 00191506040215 [Package] Contains: 00191506040208 Package: [10 Units] In Commercial Distribution |
FDA Product Code
| ODC | Endoscope channel accessory |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-05-01 |
| Device Publish Date | 2024-04-23 |
On-Brand Devices [AutoCap™ RX]
| 08714729994695 | Integrated Biopsy Cap and Guidewire Locking Device |
| 00191506000745 | Integrated Biopsy Cap and Guidewire Locking Device |
| 00191506040215 | Integrated Biopsy Cap and Guidewire Locking Device |
Trademark Results [AutoCap]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AUTOCAP 98277582 not registered Live/Pending |
Celec Enterprises 2023-11-20 |
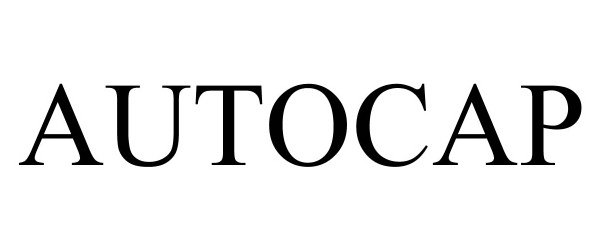 AUTOCAP 97278473 not registered Live/Pending |
Boston Scientific Scimed, Inc. 2022-02-22 |
 AUTOCAP 88143409 not registered Live/Pending |
Boston Scientific Scimed, Inc. 2018-10-04 |
 AUTOCAP 78016203 not registered Dead/Abandoned |
AutoCap, Inc. 2000-07-11 |
 AUTOCAP 77600252 3642685 Dead/Cancelled |
THOMAS & BETTS INTERNATIONAL LLC 2008-10-24 |
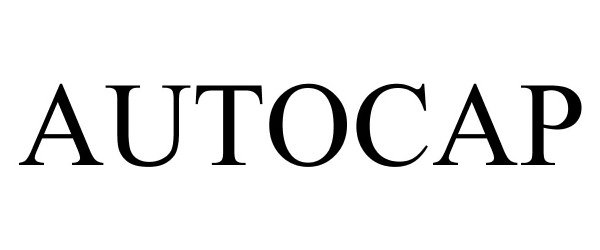 AUTOCAP 77486865 not registered Dead/Abandoned |
AUTOCAP LLC 2008-05-30 |
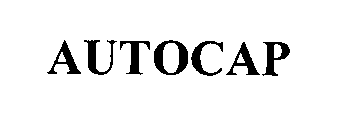 AUTOCAP 76470440 not registered Dead/Abandoned |
VERSO VERILINK, LLC F/K/A WINSLOW ASSETHOLDINGS, LLC 2002-11-25 |
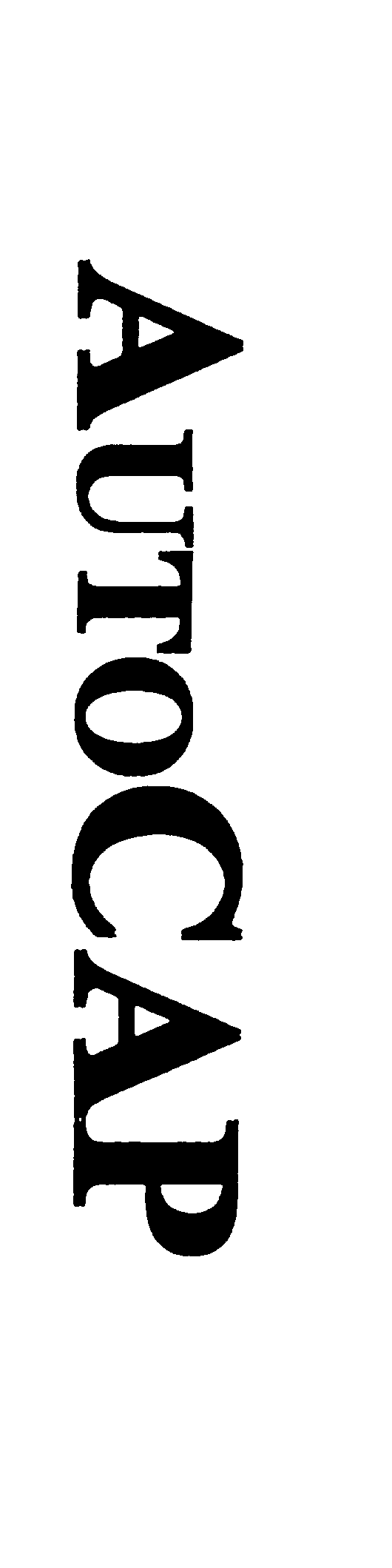 AUTOCAP 75861234 not registered Dead/Abandoned |
Visual Software Associates, Inc. 1999-11-19 |
 AUTOCAP 75223142 not registered Dead/Abandoned |
AutoCap, L.L.C. 1997-01-08 |
 AUTOCAP 74697739 1985517 Dead/Cancelled |
Pacific Scientific Company 1995-07-06 |
 AUTOCAP 73742477 1552343 Dead/Cancelled |
NATIONAL AUTOMOBILE DEALERS ASSOCIATION 1988-07-27 |
 AUTOCAP 73408189 not registered Dead/Abandoned |
NATIONAL AUTOMOBILE DEALERS ASSOCIATION 1983-01-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.