PGDx elio™ server B-01-10800
GUDID 00192721000756
PERSONAL GENOME DIAGNOSTICS INC.
Cancer-related multiple gene mutation/mRNA expression IVD, kit, nucleic acid technique (NAT)| Primary Device ID | 00192721000756 |
| NIH Device Record Key | 8d300b03-25c1-4a46-96b6-2b84da8430e2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | PGDx elio™ server |
| Version Model Number | B-01-10800 |
| Catalog Number | B-01-10800 |
| Company DUNS | 963427120 |
| Company Name | PERSONAL GENOME DIAGNOSTICS INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00192721000756 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K192063
FDA Product Code
| PZM | Next Generation Sequencing Based Tumor Profiling Test |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-05-19 |
| Device Publish Date | 2020-05-11 |
On-Brand Devices [PGDx elio™ server]
| 00192721000756 | B-01-10800 |
| 00192721001432 | PGDx elio™ server (reconditioned) |
| 00192721001456 | PGDx elio™ server (reconditioned 128C) |
| 00192721001449 | PGDx elio™ server (128C) |
Trademark Results [PGDx elio]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
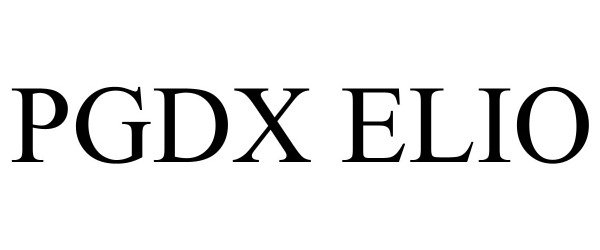 PGDX ELIO 98029210 not registered Live/Pending |
Personal Genome Diagnostics Inc. 2023-06-06 |
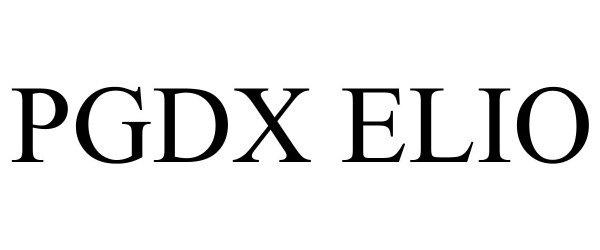 PGDX ELIO 98029209 not registered Live/Pending |
Personal Genome Diagnostics Inc. 2023-06-06 |
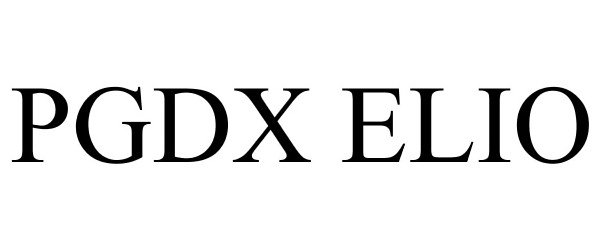 PGDX ELIO 87950371 not registered Live/Pending |
Personal Genome Diagnostics Inc. 2018-06-06 |
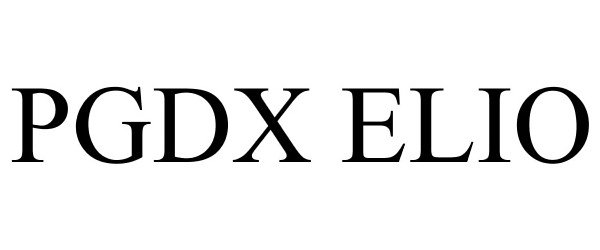 PGDX ELIO 87925167 not registered Live/Pending |
Personal Genome Diagnostics Inc. 2018-05-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.