PULSE
GUDID 00195377065513
PULSE Software, v4.0.0-bl.us
Nuvasive, Inc.
Orthopaedic stereotactic surgery system| Primary Device ID | 00195377065513 |
| NIH Device Record Key | 7abc4ca8-91a4-4e64-adcd-ab21784ea843 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | PULSE |
| Version Model Number | 1802491 |
| Company DUNS | 053950783 |
| Company Name | Nuvasive, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1(858)909-1800 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00195377065513 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K210574
FDA Product Code
| JAA | System, x-ray, fluoroscopic, image-intensified |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-05-16 |
| Device Publish Date | 2022-05-06 |
On-Brand Devices [PULSE]
| 00887517984814 | Pulse II Touchsceen Monitor |
| 00887517975065 | PULSE II System |
| 00887517973900 | Pulse Pointer, Straight Small |
| 00887517973887 | Pulse Spine Clamp, Large |
| 00887517973870 | Pulse Spine Clamp, Medium |
| 00887517973863 | Pulse Spine Clamp, Small |
| 00887517973801 | PULSE Posterior Confirmation Fixture |
| 00887517973160 | PULSE Patient Reference Array Kit |
| 00887517972309 | Pulse Camera Cart |
| 00887517953469 | PULSE Camera |
| 00887517988065 | PULSE II Flat Array, A |
| 00887517988058 | PULSE II Flat Array, B |
| 00887517988041 | PULSE II Flat Array, C |
| 00887517973894 | Pulse Driver, Pin |
| 00887517814128 | PULSE Cart Power and Data Cable |
| 00887517996138 | PULSE Adapter, VGA to DVI |
| 00887517996121 | PULSE Adapter, BNC to DVI |
| 00887517990808 | PULSE Patient Module 4 |
| 00887517990792 | PULSE III System |
| 00887517986528 | PULSE II Cable, Ethernet |
| 00887517939807 | PULSE SSEP Pod |
| 00887517011541 | Pulse System |
| 00887517011534 | Pulse IOM Set |
| 00887517001627 | PULSE Cable, BNC |
| 00887517990785 | PULSE Navigation Instrument Set, BETA |
| 00887517990778 | PULSE II System, BETA |
| 00887517990761 | PULSE Camera Cart, BETA |
| 00887517990754 | PULSE Camera Set, BETA |
| 00887517955319 | PULSE Control Unit DVI Adapter |
| 00887517945822 | PULSE Monitor Cable, Shippable |
| 00887517945815 | PULSE CPU Power Cable, Shippable |
| 00887517931771 | PULSE Control Unit, Shippable |
| 00887517834157 | PULSE Patient Module 4 |
| 00887517994141 | PULSE Flat Array C, Beta |
| 00887517994134 | PULSE Flat Array B, Beta |
| 00887517994127 | PULSE Flat Array A, Beta |
| 00887517991911 | PULSE TMAP Kit |
| 00887517960344 | Pulse Shippable Monitor Set - ALPHA |
| 00887517960337 | Pulse Shippable Control Unit Set - ALPHA |
| 00887517960320 | Pulse Shippable Cart Set - ALPHA |
| 00887517939876 | MIOM Electrode Kit, Surface |
| 00887517939869 | MIOM Electrode Kit, Needle |
| 00887517939852 | PULSE MEP Surface Electrodes, 2 Pack |
| 00887517939845 | PULSE MEP Needle Electrodes, 2 Pack |
| 00887517939838 | PULSE SSEP Surface Electrodes |
| 00887517939821 | PULSE SSEP Needle Electrodes |
| 00887517824578 | Multimodality Harness |
| 00887517824554 | EMG Electrode Kit, Surface |
| 00887517824530 | EMG Electrode Kit, Needle |
| 00887517824523 | Stimulation Clip-Activator, Single |
Trademark Results [PULSE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PULSE 98853486 not registered Live/Pending |
Pulse Trading Cards LLC 2024-11-14 |
 PULSE 98838317 not registered Live/Pending |
SUPERNOVA INDUSTRIES CORP. 2024-11-05 |
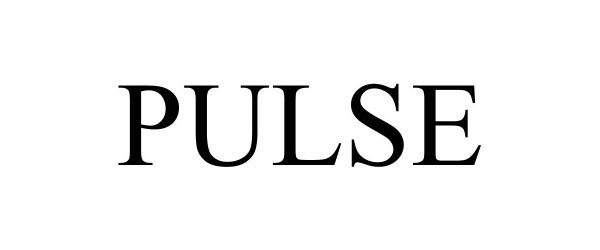 PULSE 98526642 not registered Live/Pending |
Medalogix LLC 2024-04-30 |
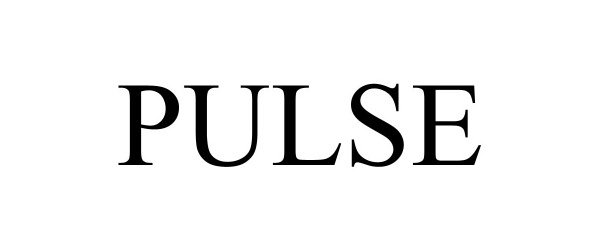 PULSE 98521450 not registered Live/Pending |
MailSPEC Incorporated 2024-04-26 |
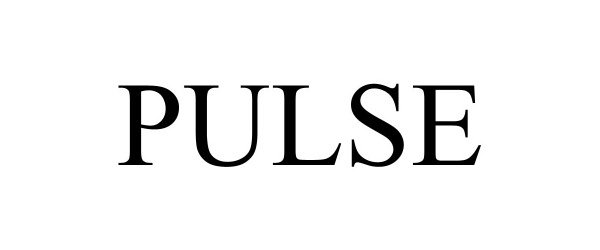 PULSE 98517287 not registered Live/Pending |
vcfo Holdings, Inc. 2024-04-24 |
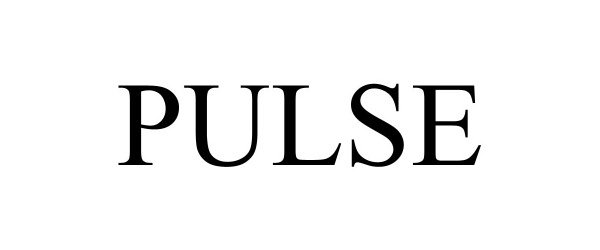 PULSE 98314293 not registered Live/Pending |
DLD Water, LLC 2023-12-14 |
 PULSE 98305698 not registered Live/Pending |
Pulse Trading Cards LLC 2023-12-08 |
 PULSE 98298153 not registered Live/Pending |
Ncardia B.V. 2023-12-04 |
 PULSE 98198903 not registered Live/Pending |
Khaziri, David 2023-09-27 |
 PULSE 98198903 not registered Live/Pending |
Khaziri, William 2023-09-27 |
 PULSE 98061547 not registered Live/Pending |
TekFive, Inc. 2023-06-27 |
 PULSE 97917404 not registered Live/Pending |
Pulse Labs, Inc. 2023-05-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.