AcrySof® ACRYSERT® SN60WS.265
GUDID 00380655097595
AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FILTER, 13.0mm LENGTH, 6.0mm ANTERIORASYMMETRIC BICONVEX OPTIC, PLANAR HAPTICS.
Alcon Laboratories, Inc.
Posterior-chamber intraocular lens, pseudophakic| Primary Device ID | 00380655097595 |
| NIH Device Record Key | 731bee57-f6a9-4886-8a3c-ec50e99cdb17 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | AcrySof® ACRYSERT® |
| Version Model Number | SN60WS |
| Catalog Number | SN60WS.265 |
| Company DUNS | 008018525 |
| Company Name | Alcon Laboratories, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com | |
| Phone | +1(800)862-5266 |
| web.masterus@alcon.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00380655097595 [Primary] |
FDA Product Code
| HQL | intraocular lens |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2015-10-24 |
On-Brand Devices [AcrySof® ACRYSERT®]
| 00380655097663 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097656 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097649 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097632 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097625 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097618 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097601 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097595 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097588 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097571 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097564 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097557 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097540 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097533 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097526 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097519 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097502 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097496 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097489 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097472 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097465 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097458 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097441 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097434 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097427 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097410 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097403 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097397 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097380 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097373 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097366 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097359 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097342 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097335 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097328 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097311 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097304 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097298 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097281 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097274 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097267 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097250 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097243 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097236 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097229 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097212 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097205 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097199 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380655097182 | AcrySof(R) IQ ASPHERIC Natural SP IOL WITHTHE AcrySert(R) DELIVERY SYSTEM, UV WITH BLUELIGHT FIL |
| 00380652249416 | AcrySof(R) IQ ASPHERIC IOL, SP ACRYLIC FOLDABLELENS, w/AcrySert(R) DELIVERY SYSTEM, UV w/BLUELIG |
Trademark Results [AcrySof]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACRYSOF 87222195 5209388 Live/Registered |
Novartis AG 2016-11-01 |
 ACRYSOF 85920056 not registered Dead/Abandoned |
Novartis AG 2013-05-01 |
 ACRYSOF 85068866 3908096 Live/Registered |
NOVARTIS AG 2010-06-22 |
 ACRYSOF 78877203 3213231 Dead/Cancelled |
ALCON RESEARCH, LTD. 2006-05-05 |
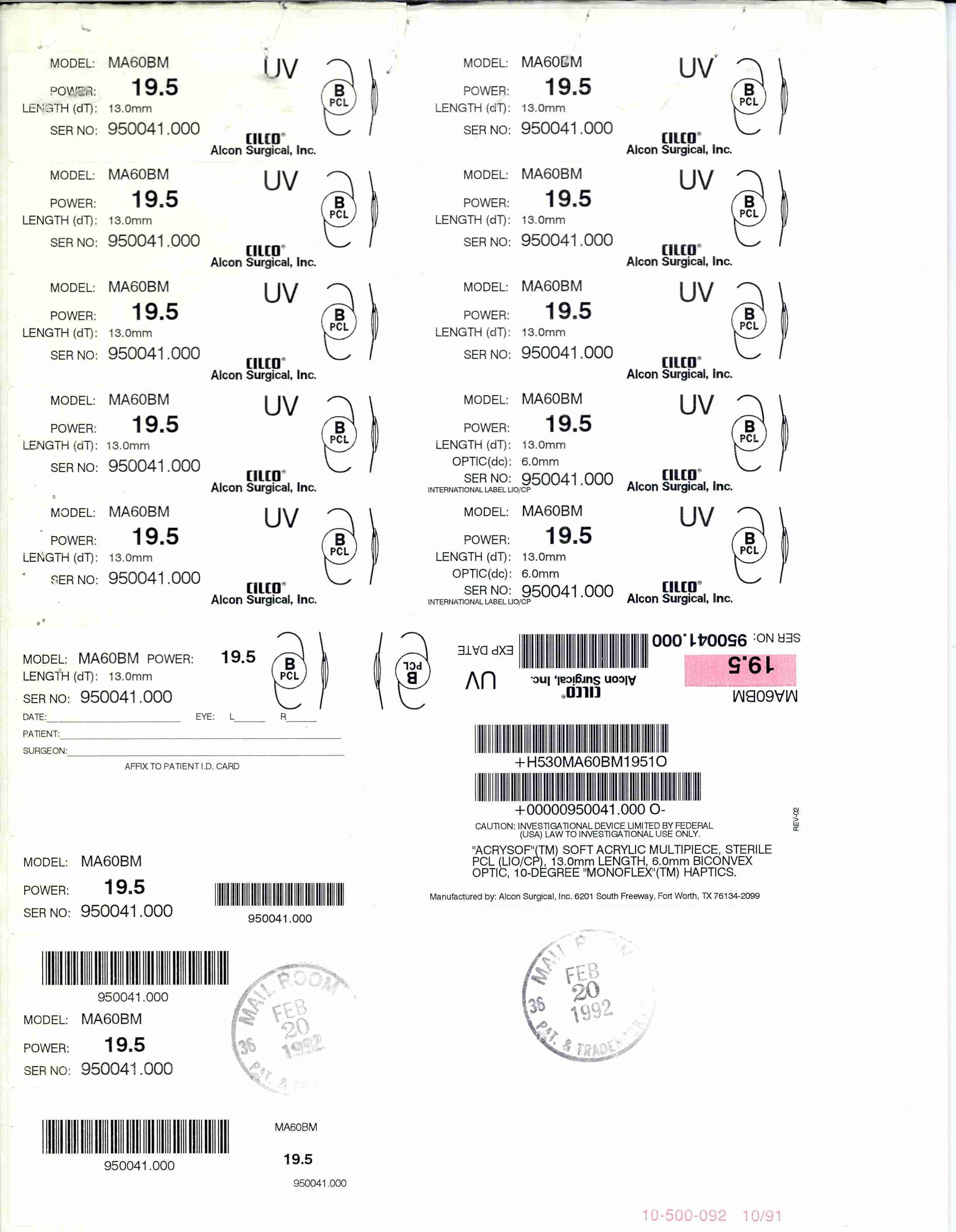 ACRYSOF 74129590 1705808 Live/Registered |
ALCON RESEARCH, LLC 1991-01-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.