Rapport
GUDID 00384702009012
VTD Rapport Pump Handle in a bag
OWEN MUMFORD USA INCORPORATED
Penile vacuum device| Primary Device ID | 00384702009012 |
| NIH Device Record Key | 86be0864-790c-47e5-91bf-f5c5db929482 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Rapport |
| Version Model Number | SM 2009 |
| Company DUNS | 803401454 |
| Company Name | OWEN MUMFORD USA INCORPORATED |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00384702009012 [Primary] |
FDA Product Code
| LKY | Device, External Penile Rigidity |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-06-28 |
| Device Publish Date | 2023-06-20 |
Devices Manufactured by OWEN MUMFORD USA INCORPORATED
| 00384703160019 - Empelvic | 2025-09-08 Pelvic Floor Trainer - Femail |
| 00384703150010 - Empelvic | 2025-09-01 Pelvic Floor Trainer - Femail |
| 00384703155015 - Empelvic | 2025-09-01 Pelvic Floor Trainer - Male |
| 00761059640028 - Unifine OTC | 2025-02-24 Unifine OTC Pen Needle 4mm x 32G 100 ct |
| 00761059641032 - Unifine OTC | 2025-02-24 Unifine OTC Pen Needles 4mm x 32G 50 ct |
| 00761059650027 - Unifine OTC | 2025-02-24 Unifine OTC Pen Needle, 5mm x 31G, 100 ct |
| 00761059651031 - Unifine OTC | 2025-02-24 Unifine OTC Pen Needle, 5mm x 31G, 50 ct |
| 00761059354024 - Unifine Pentips | 2024-02-13 Auto Control 1st Tier Unifine Pentips pen needles - 4mm x 32G |
Trademark Results [Rapport]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RAPPORT 98854082 not registered Live/Pending |
Jacques, Mauricio 2024-11-14 |
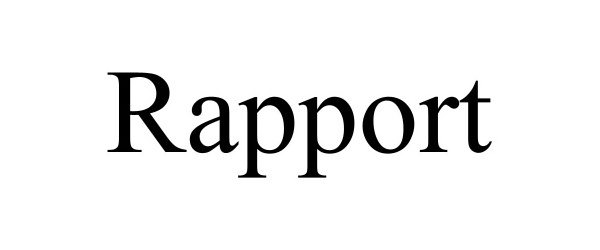 RAPPORT 97977341 not registered Live/Pending |
Rapport Inc. 2022-06-09 |
 RAPPORT 97630353 not registered Live/Pending |
Rapport Therapeutics, Inc. 2022-10-13 |
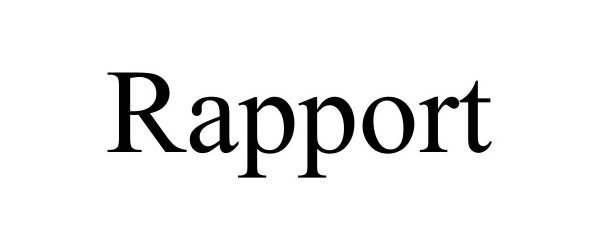 RAPPORT 97552448 not registered Live/Pending |
Covetrus Software Services, LLC 2022-08-17 |
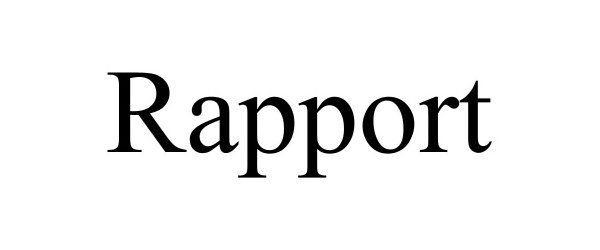 RAPPORT 97448582 not registered Live/Pending |
Rapport Inc. 2022-06-09 |
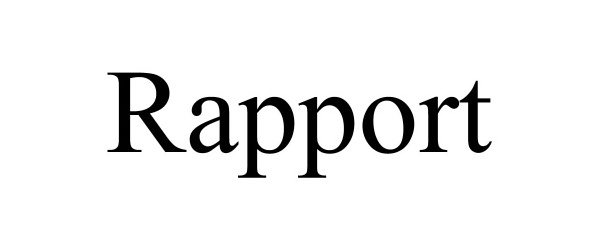 RAPPORT 90632657 not registered Live/Pending |
Alexander Cole Acosta 2021-04-08 |
 RAPPORT 90013909 not registered Live/Pending |
Afable, Victor V., 2020-06-22 |
 RAPPORT 88214899 5781715 Live/Registered |
Nufarm Americas Inc. 2018-12-03 |
 RAPPORT 88207202 5823485 Live/Registered |
MSH, Inc. 2018-11-27 |
 RAPPORT 87728815 not registered Dead/Abandoned |
Rapport, Inc. 2017-12-20 |
 RAPPORT 87670196 5591707 Live/Registered |
RehabPlus Staffing Group, Inc. 2017-11-02 |
 RAPPORT 87073388 5307658 Live/Registered |
Compass Group Holdings PLC 2016-06-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.