Strive®
GUDID 00604351966109
MONAGHAN MEDICAL CORPORATION
Peak flow meter, manual| Primary Device ID | 00604351966109 |
| NIH Device Record Key | bd14cbd3-ffb8-4ae1-80da-2219b3505e49 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Strive® |
| Version Model Number | Peak Flow Meter, cs/10 |
| Company DUNS | 056332380 |
| Company Name | MONAGHAN MEDICAL CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 518-561-7330 |
| customerservice@monaghanmed.com | |
| Phone | 518-561-7330 |
| customerservice@monaghanmed.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00604351966109 [Primary] |
| GS1 | 50604351966104 [Package] Package: [10 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K123945
FDA Product Code
| BZH | Meter, Peak Flow, Spirometry |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-20 |
Devices Manufactured by MONAGHAN MEDICAL CORPORATION
| 00604351506121 - Aerochamber2go Anti-Static Valved Holding Chamber | 2025-06-09 AEROCHAMBER2GO CHAMBER, RETAIL |
| 50604351707066 - Nasoneb® | 2024-11-04 NasoNeb Sinus Therapy System cs/6 |
| 50604351505129 - Aerobika,OPEP | 2024-05-24 AEROBIKA, OPEP DEVICE, RETAIL- 50512 |
| 50604351504122 - Aerochamber Plus® Flow-Vu® | 2024-03-11 AC Plus, Flow-Vu aVHC, Intermediate Mask, Retail - 50412 |
| 00604351500129 - Aerochamber Plus® Flow-Vu® | 2023-11-22 AC+FV aVHC Mouthpiece Retail - 50012 |
| 50604351501121 - Aerochamber Plus® Flow-Vu® | 2023-11-22 AC Plus, Flow-Vu aVHC, Small Mask, Retail - 50112 |
| 50604351502128 - Aerochamber Plus® Flow-Vu® | 2023-11-22 AC Plus, Flow-Vu aVHC, Medium Mask, Retail - 50212 |
| 50604351503125 - Aerochamber Plus® Flow-Vu® | 2023-11-22 AC Plus, Flow-Vu aVHC, Large Mask, Retail - 50312 |
Trademark Results [Strive]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STRIVE 98688283 not registered Live/Pending |
BRAND CONTROL LTD 2024-08-08 |
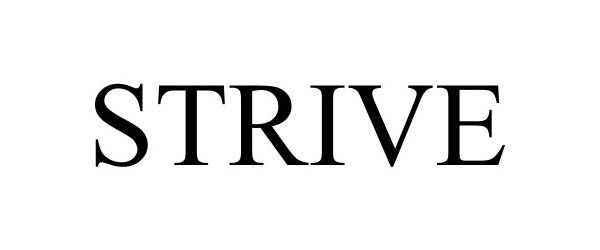 STRIVE 98491324 not registered Live/Pending |
Strive Nutrition Corp. 2024-04-09 |
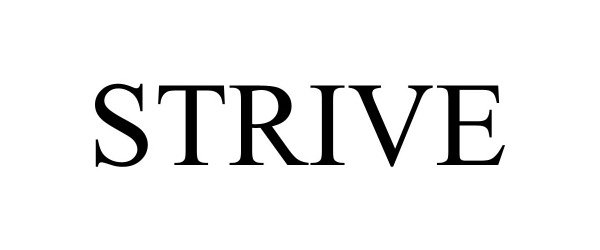 STRIVE 98197666 not registered Live/Pending |
Hive Cocktail, Inc. 2023-09-26 |
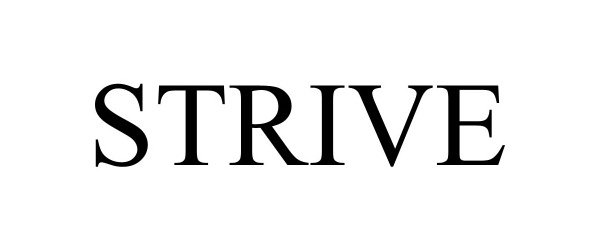 STRIVE 98090425 not registered Live/Pending |
Embecta Corp. 2023-07-18 |
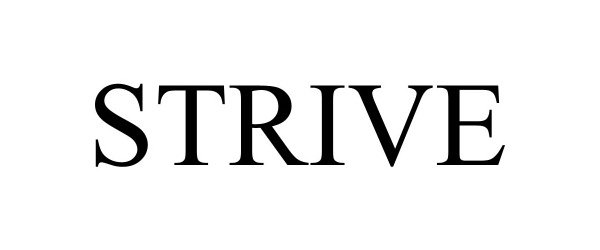 STRIVE 98015704 not registered Live/Pending |
Universal Engineering Sciences, LLC 2023-05-26 |
 STRIVE 97852721 not registered Live/Pending |
Olavarria, Angel L 2023-03-23 |
 STRIVE 97689224 not registered Live/Pending |
STRIVE SOFTWARE 2022-11-22 |
 STRIVE 97689198 not registered Live/Pending |
STRIVE SOFTWARE 2022-11-22 |
 STRIVE 97469362 not registered Live/Pending |
CIRCLE SPECIALTY INC. 2022-06-21 |
 STRIVE 97437851 not registered Live/Pending |
Strive Enterprises, Inc. 2022-06-01 |
 STRIVE 97426379 not registered Live/Pending |
Strive Enterprises, Inc. 2022-05-24 |
 STRIVE 97398960 not registered Live/Pending |
Strive Creations, LLC 2022-05-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.