UNIVERSAL F2® F01
GUDID 00612649151410
F2 TRANSPORT VALVE
King Systems Corporation
Anaesthesia breathing circuit, single-use| Primary Device ID | 00612649151410 |
| NIH Device Record Key | 912862e2-2c46-417f-8ee6-ae0ca2fe1d52 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | UNIVERSAL F2® |
| Version Model Number | F01 |
| Catalog Number | F01 |
| Company DUNS | 009299017 |
| Company Name | King Systems Corporation |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00612649151410 [Package] Contains: 00612649202686 Package: [30 Units] In Commercial Distribution |
| GS1 | 00612649202686 [Primary] |
FDA Product Code
| CAI | Circuit, breathing (w connector, adaptor, y piece) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-02-17 |
| Device Publish Date | 2023-02-09 |
On-Brand Devices [UNIVERSAL F2®]
| 00612649151410 | F2 TRANSPORT VALVE |
| 00612649141008 | UNIVERSAL F2 EXTENSION SET |
| 00612649140995 | UNIVERSAL F2 EXTENSION SET |
| 00612649154527 | UNIVERSAL F2 EXTENSION SET |
| 00612649166353 | UNIVERSAL F2 CIRCUIT |
| 00612649150444 | UNIVERSAL F2 CIRCUIT |
| 00612649164885 | UNIVERSAL F2 CIRCUIT |
| 00612649145006 | UNIVERSAL F2 CIRCUIT |
| 00612649143644 | UNIVERSAL F2 CIRCUIT |
| 00612649142616 | UNIVERSAL F2 CIRCUIT |
| 00612649142593 | UNIVERSAL F2 CIRCUIT |
Trademark Results [UNIVERSAL F2]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
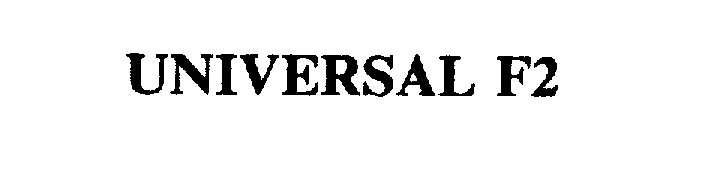 UNIVERSAL F2 75354132 2336533 Live/Registered |
King Systems Corporation 1997-09-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.