BRYAN® Cervical Disc System
GUDID 00613994181237
MOTION 6470317 17MM BRYAN CRV DSC PRSTHS
MEDTRONIC SOFAMOR DANEK, INC.
Cervical total disc replacement prosthesis, sterile| Primary Device ID | 00613994181237 |
| NIH Device Record Key | 47f75d91-3d35-4f23-b4bf-e930e1801fef |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | BRYAN® Cervical Disc System |
| Version Model Number | 6470317 |
| Company DUNS | 830350380 |
| Company Name | MEDTRONIC SOFAMOR DANEK, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
| Outer Diameter | 17 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00613994181237 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P060023
FDA Product Code
| MJO | PROSTHESIS, INTERVERTEBRAL DISC |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2014-09-23 |
On-Brand Devices [BRYAN® Cervical Disc System]
| 00613994365354 | DISC 6478418 MILLING 18MM |
| 00613994365347 | DISC 6478417 MILLING 17MM |
| 00613994365330 | DISC 6478416 MILLING 16MM |
| 00613994365323 | DISC 6478415 MILLING 15MM |
| 00613994365316 | DISC 6478414 MILLING 14MM |
| 00613994363824 | CUTTER 6474524 14MM |
| 00613994363817 | CUTTER 6474525 15MM |
| 00613994363800 | CUTTER 6474526 16MM |
| 00613994363794 | CUTTER 6474527 17MM |
| 00613994363787 | CUTTER 6474528 18MM |
| 00613994363640 | TRIAL 6474058 SIZER 18MM |
| 00613994363633 | TRIAL 6474057 SIZER 17MM |
| 00613994363626 | TRIAL 6474056 SIZER 16MM |
| 00613994363619 | TRIAL 6474055 SIZER 15MM |
| 00613994363602 | TRIAL 6474054 SIZER 14MM |
| 00613994360717 | INSERTER 6473610 IMPLANT |
| 00613994181244 | MOTION 6470318 18MM BRYAN CRV DSC PRSTHS |
| 00613994181237 | MOTION 6470317 17MM BRYAN CRV DSC PRSTHS |
| 00613994181220 | MOTION 6470316 16MM BRYAN CRV DSC PRSTHS |
| 00613994181213 | MOTION 6470315 15MM BRYAN CRV DSC PRSTHS |
| 00613994181206 | MOTION 6470314 14MM BRYAN CRV DSC PRSTHS |
Trademark Results [BRYAN]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRYAN 98718865 not registered Live/Pending |
Bryansito LLC 2024-08-27 |
 BRYAN 88513626 not registered Live/Pending |
Tart Optical Enterprises, LLC 2019-07-15 |
 BRYAN 87511631 not registered Dead/Abandoned |
RAINMAKER INTERNATIONAL LIMITED 2017-06-29 |
 BRYAN 85839959 4929947 Live/Registered |
Bryan University, LLC 2013-02-04 |
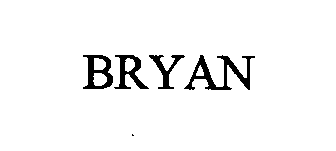 BRYAN 76352789 2683009 Live/Registered |
BURNHAM SERVICES, INC. 2001-12-26 |
 BRYAN 75881705 not registered Dead/Abandoned |
Bampenchow, Wanchai 1999-12-27 |
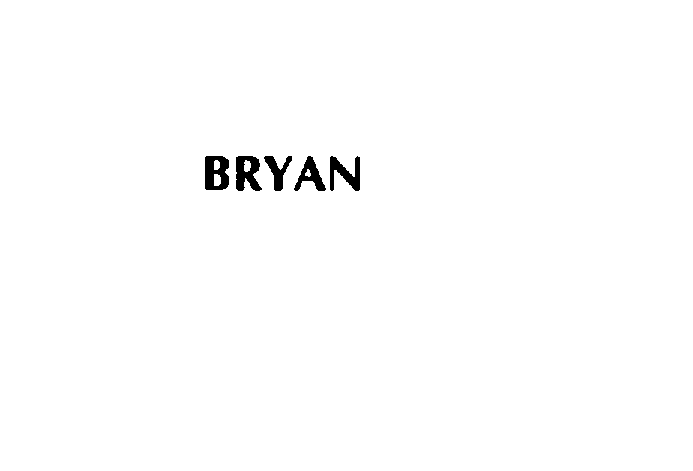 BRYAN 75772987 2643395 Live/Registered |
WARSAW ORTHOPEDIC, INC. 1999-08-10 |
 BRYAN 75394787 2216333 Live/Registered |
SARA LEE FOODS, LLC 1997-11-24 |
 BRYAN 74622502 2138110 Dead/Cancelled |
SARAMAR CORPORATION 1995-01-18 |
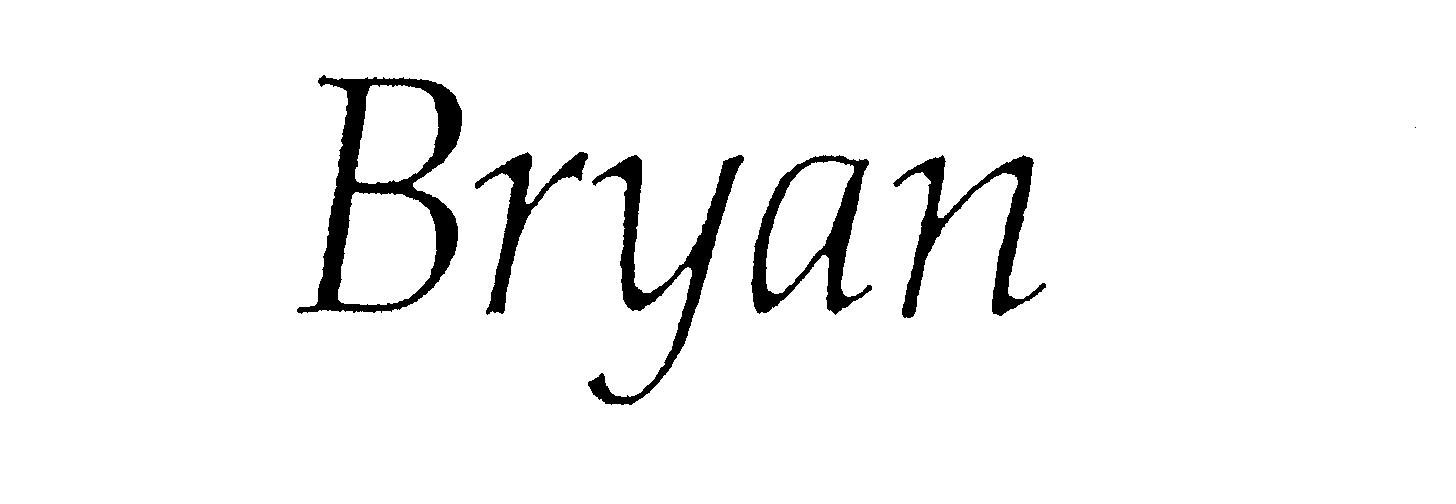 BRYAN 74611630 1979338 Live/Registered |
BRYAN APPAREL CORP. 1994-12-16 |
 BRYAN 74533748 1902897 Dead/Cancelled |
SARA LEE FOODS, INC. 1994-06-06 |
 BRYAN 74364019 1813931 Dead/Cancelled |
SARA LEE CORPORATION 1993-03-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.