Exchange Select
GUDID 00614299396432
Exchange Select One Step Ovulation Predictor
SPD SWISS PRECISION DIAGNOSTICS GMBH
Natural conception assistance kit| Primary Device ID | 00614299396432 |
| NIH Device Record Key | b1f73251-1090-4b3d-ac70-94a5469c3fd1 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Exchange Select |
| Version Model Number | One Step Ovulation Predictor |
| Company DUNS | 483609579 |
| Company Name | SPD SWISS PRECISION DIAGNOSTICS GMBH |
| Device Count | 7 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 1-800-899-7353 |
| xx@xx.xx | |
| Phone | 1-800-899-7353 |
| xx@xx.xx | |
| Phone | 1-800-899-7353 |
| xx@xx.xx | |
| Phone | 1-800-899-7353 |
| xx@xx.xx | |
| Phone | 1-800-899-7353 |
| xx@xx.xx | |
| Phone | 1-800-899-7353 |
| xx@xx.xx | |
| Phone | 1-800-899-7353 |
| xx@xx.xx |
Operating and Storage Conditions
| Storage Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00614299396432 [Primary] |
| GS1 | 00633472000140 [Unit of Use] |
| GS1 | 10614299396439 [Package] Package: Shipping case [6 Units] In Commercial Distribution |
FDA Product Code
| CEP | Radioimmunoassay, Luteinizing Hormone |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2019-03-11 |
| Device Publish Date | 2019-03-01 |
On-Brand Devices [Exchange Select ]
| 00614299396432 | Exchange Select One Step Ovulation Predictor |
| 10614299437385 | Exchange Select DPS +/-Blue Cap |
Trademark Results [Exchange Select]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 EXCHANGE SELECT 87934653 not registered Live/Pending |
Army and Air Force Exchange Service 2018-05-24 |
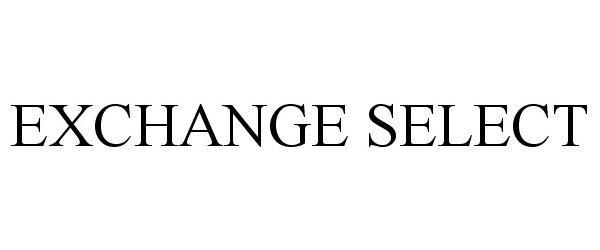 EXCHANGE SELECT 78675226 3155373 Dead/Cancelled |
Navy Exchange Service Command ("NEXCOM") 2005-07-21 |
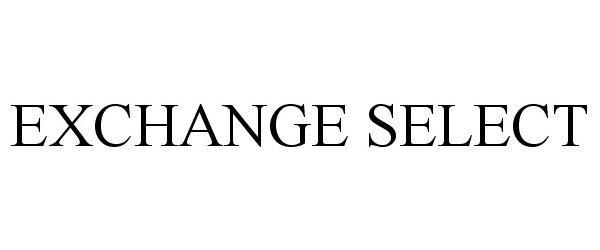 EXCHANGE SELECT 78675226 3155373 Dead/Cancelled |
Army and Air Force Exchange Service 2005-07-21 |
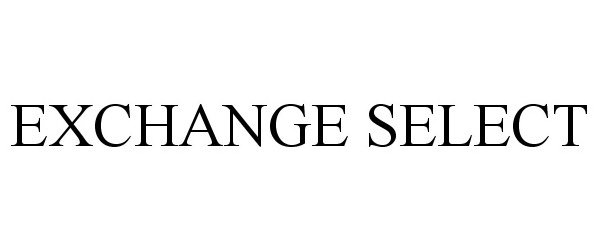 EXCHANGE SELECT 78675098 3155372 Dead/Cancelled |
Navy Exchange Service Command ("NEXCOM") 2005-07-21 |
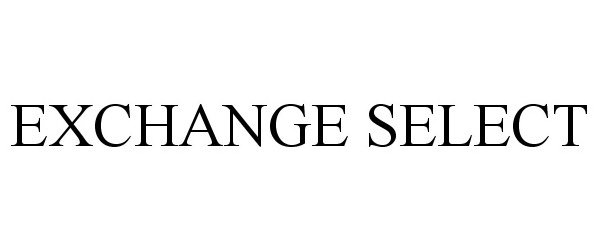 EXCHANGE SELECT 78675098 3155372 Dead/Cancelled |
Army and Air Force Exchange Service 2005-07-21 |
 EXCHANGE SELECT 78117460 not registered Dead/Abandoned |
Army and Air Force Exchange Service 2002-03-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.