Accu-Cut® SRM™ 200 1441
GUDID 00615233012975
Protective Dust Cover
SAKURA FINETEK U.S.A., INC.
Instrument/analyser test tube rack cover IVD| Primary Device ID | 00615233012975 |
| NIH Device Record Key | 74149e1f-6b53-4ca2-bdb8-009119d890b8 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Accu-Cut® SRM™ 200 |
| Version Model Number | 1441 |
| Catalog Number | 1441 |
| Company DUNS | 179135769 |
| Company Name | SAKURA FINETEK U.S.A., INC. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(310)972-7800 |
| TS@sakuraus.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00615233012975 [Primary] |
FDA Product Code
| IDO | MICROTOME, ROTARY |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-02-14 |
| Device Publish Date | 2020-02-06 |
On-Brand Devices [Accu-Cut® SRM™ 200]
| 00615233012975 | Protective Dust Cover |
| 00615233012906 | Rotary Microtome (Non-retracting) |
| 00615233012890 | Rotary Microtome (Retracting) |
| 00615233012944 | Low-Profile Back Plate (part of item 1435) |
| 00615233012937 | High-Profile Back Plate (part of item 1469) |
| 08720177655170 | Rotary Microtome (Retracting) |
Trademark Results [Accu-Cut]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
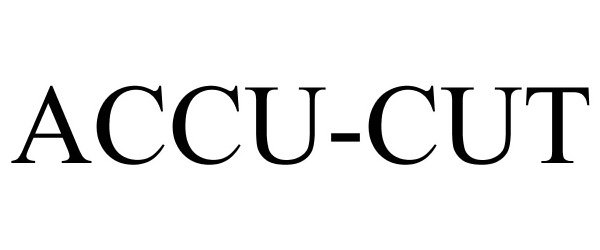 ACCU-CUT 77096552 3316453 Dead/Cancelled |
Winsource Industries LTD. 2007-02-01 |
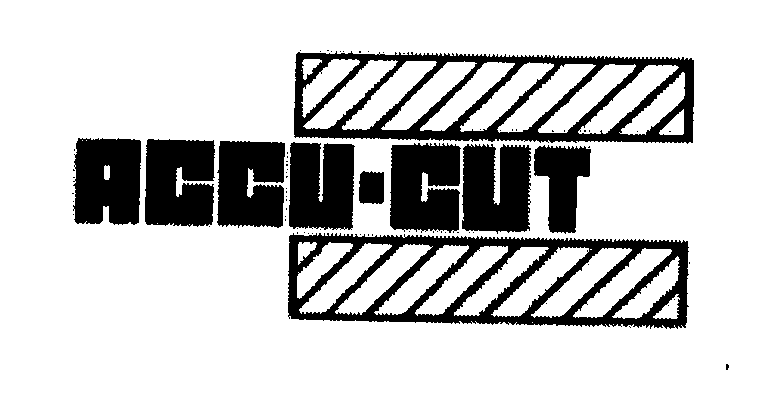 ACCU-CUT 76503925 2873678 Live/Registered |
Accu-Cut Diamond Bore sizing Systems, Inc. 2003-04-04 |
 ACCU-CUT 76503925 2873678 Live/Registered |
Accu-Cut Diamond Tool Company, Inc. 2003-04-04 |
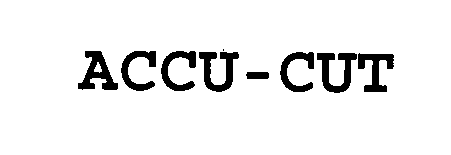 ACCU-CUT 76323994 3250089 Live/Registered |
Brockie International, Inc. 2001-10-12 |
 ACCU-CUT 76013931 2568218 Live/Registered |
BIOPRO, INC. 2000-03-30 |
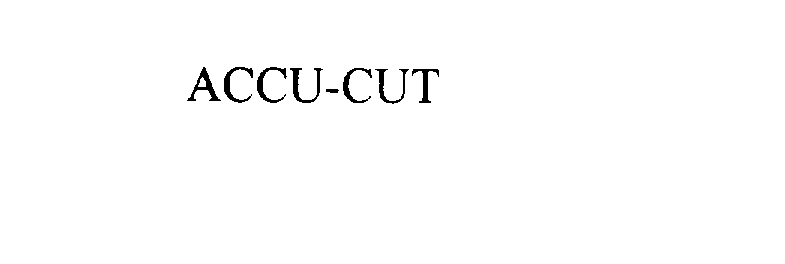 ACCU-CUT 76005051 2959992 Live/Registered |
STANLEY BLACK & DECKER, INC. 2000-03-20 |
 ACCU-CUT 76004328 not registered Dead/Abandoned |
Brockie International, Inc. 2000-03-20 |
 ACCU-CUT 75626349 not registered Dead/Abandoned |
EBI Medical Systems, Inc. 1999-01-19 |
 ACCU-CUT 75560579 not registered Dead/Abandoned |
Brockie International, Inc 1998-09-28 |
 ACCU-CUT 75449654 2379912 Dead/Cancelled |
SUFIX USA, INC. 1998-03-13 |
 ACCU-CUT 75089151 not registered Dead/Abandoned |
Accu-Cut Diamond Tool Co., Inc. 1996-04-05 |
 ACCU-CUT 74508646 not registered Dead/Abandoned |
McKeegan Equipment & Supply 1994-04-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.