CALCIUM SK140-10
GUDID 00628063400270
SEKISUI DIAGNOSTICS, LLC
Calcium (Ca2+) IVD, kit, spectrophotometry| Primary Device ID | 00628063400270 |
| NIH Device Record Key | d0a3fb73-a47f-4afb-9a65-05360c11c82f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | CALCIUM |
| Version Model Number | SK140-10 |
| Catalog Number | SK140-10 |
| Company DUNS | 966812344 |
| Company Name | SEKISUI DIAGNOSTICS, LLC |
| Device Count | 2 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00628063400270 [Primary] |
| GS1 | 10628063400277 [Unit of Use] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K895873
FDA Product Code
| CJY | AZO DYE, CALCIUM |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-20 |
Devices Manufactured by SEKISUI DIAGNOSTICS, LLC
| 10742860100670 - OSOM COVID-19 Test | 2026-01-27 OSOM COVID-19 Test |
| 10742860100052 - OSOM BV Blue 25T Kit | 2026-01-06 |
| 00742860100062 - OSOM BV Blue Control Kit | 2026-01-06 |
| 00742860000065 - OSOM Flu SARS-CoV-2 Combo Test | 2025-12-23 OSOM Flu SARS-CoV-2 Combo Test |
| 10742860100663 - OSOM RSV Test Home | 2025-05-27 OSOM RSV Test |
| 00628063401291 - Acetaminophen | 2023-05-16 |
| 00628063401307 - Acetaminophen Calibrator | 2023-05-16 |
| 00628063401314 - Alinity c Acetaminophen | 2023-05-16 |
Trademark Results [CALCIUM]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
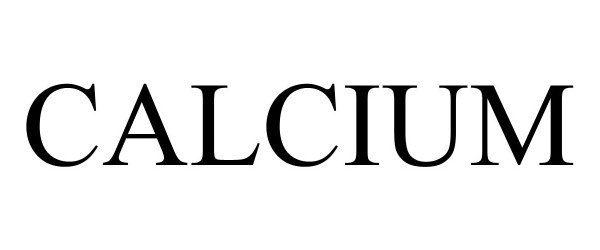 CALCIUM 98423472 not registered Live/Pending |
Senseeker Engineering Inc. 2024-02-27 |
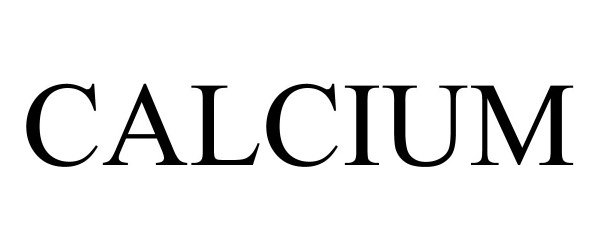 CALCIUM 97134337 not registered Live/Pending |
HEALTHCHAMPION PARTNERS, LLC 2021-11-19 |
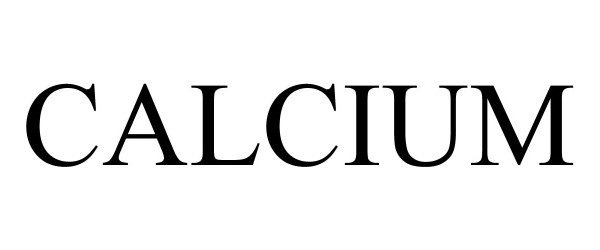 CALCIUM 90749557 not registered Live/Pending |
Senseeker Engineering Inc. 2021-06-02 |
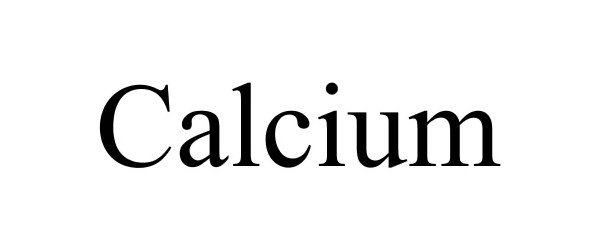 CALCIUM 87085728 5337976 Live/Registered |
CALCIUM HOLDINGS LIMITED 2016-06-28 |
 CALCIUM 87073093 5337964 Live/Registered |
CALCIUM HOLDINGS LIMITED 2016-06-15 |
 CALCIUM 75351400 2176942 Dead/Cancelled |
Tropicana Products, Inc. 1997-09-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.