QUALITY CHOICE
GUDID 00635515966918
QUALITY CHOICE CONTACT LENS CASE - 2 COUNT
SACKS HOLDINGS, INC.
Contact lens case| Primary Device ID | 00635515966918 |
| NIH Device Record Key | 96f8f574-a20b-4891-a334-ab2af166d638 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | QUALITY CHOICE |
| Version Model Number | 00635515966918 |
| Company DUNS | 047929043 |
| Company Name | SACKS HOLDINGS, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com | |
| Phone | 8587201100 |
| dchodorow@sacksholdings.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00635515966918 [Primary] |
FDA Product Code
| LRX | Case, Contact Lens |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-04-05 |
| Device Publish Date | 2024-03-28 |
Devices Manufactured by SACKS HOLDINGS, INC.
| 00011822318068 - RITE AID | 2024-04-05 RA CONTACT LENS CASE 6 PACK |
| 00635515966918 - QUALITY CHOICE | 2024-04-05QUALITY CHOICE CONTACT LENS CASE - 2 COUNT |
| 00635515966918 - QUALITY CHOICE | 2024-04-05 QUALITY CHOICE CONTACT LENS CASE - 2 COUNT |
Trademark Results [QUALITY CHOICE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
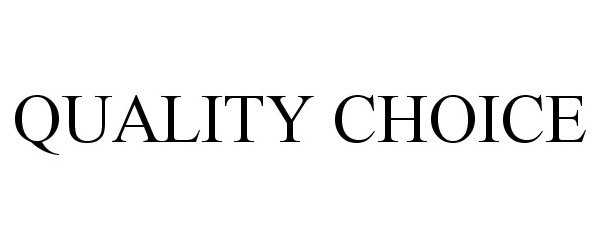 QUALITY CHOICE 77326160 3440520 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 2007-11-09 |
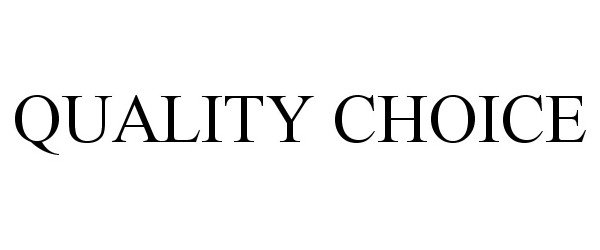 QUALITY CHOICE 77326104 3440519 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 2007-11-09 |
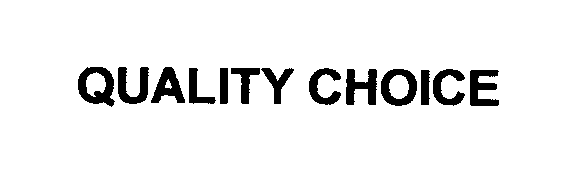 QUALITY CHOICE 76571887 2964690 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 2004-01-26 |
 QUALITY CHOICE 74561878 2144847 Live/Registered |
CHAIN DRUG MARKETING ASSOCIATION, INC. 1994-08-17 |
 QUALITY CHOICE 74035467 not registered Dead/Abandoned |
Manhattan Life Insurance Co., The 1990-03-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.