Durasafe
GUDID 00640562123023
Vinyl powder free examination gloves 2302, small
Durasafe Inc.
Vinyl examination/treatment glove, non-powdered| Primary Device ID | 00640562123023 |
| NIH Device Record Key | 2ce3cbaa-3da5-47a1-bfbd-1a327878d1b7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Durasafe |
| Version Model Number | 2302 |
| Company DUNS | 061046707 |
| Company Name | Durasafe Inc. |
| Device Count | 100 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00640562023026 [Primary] |
| GS1 | 00640562123023 [Package] Contains: 00640562023026 Package: Case [10 Units] In Commercial Distribution |
| GS1 | 10640562023023 [Unit of Use] |
FDA Product Code
| LYZ | Vinyl Patient Examination Glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-10-30 |
| Device Publish Date | 2023-10-20 |
On-Brand Devices [Durasafe]
| 00640562123054 | Vinyl powder free examination gloves 2305 x-large |
| 00640562023040 | Vinyl powder free examination gloves 2304 large |
| 00640562123030 | Vinyl powder free examination gloves 2303 medium |
| 00640562123023 | Vinyl powder free examination gloves 2302, small |
Trademark Results [Durasafe]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DURASAFE 85707507 4275917 Dead/Cancelled |
Benumof, Benjamin 2012-08-20 |
 DURASAFE 85707507 4275917 Dead/Cancelled |
Wilson, Rick 2012-08-20 |
 DURASAFE 78385549 3124396 Dead/Cancelled |
AMARR COMPANY 2004-03-16 |
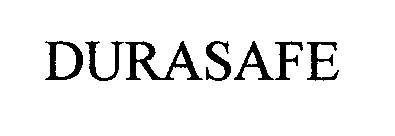 DURASAFE 76470962 not registered Dead/Abandoned |
ATS Products, Inc. 2002-11-29 |
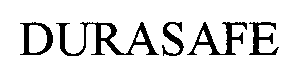 DURASAFE 76452394 3280272 Live/Registered |
Sof Surfaces Inc. 2002-09-23 |
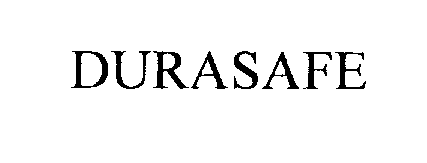 DURASAFE 76301347 2682707 Live/Registered |
DURASAFE INCORPORATED 2001-08-20 |
 DURASAFE 75822148 2444027 Dead/Cancelled |
MOHAWK CARPET DISTRIBUTION, INC 1999-10-13 |
 DURASAFE 75039444 not registered Dead/Abandoned |
Southern Pacific Coast Corp. 1996-01-02 |
 DURASAFE 74557084 1907232 Live/Registered |
Becton, Dickinson and Company 1994-08-04 |
 DURASAFE 74030051 not registered Dead/Abandoned |
American Thermal Corporation 1990-02-20 |
 DURASAFE 73454589 1304699 Dead/Cancelled |
Meilink Industries, Inc. 1983-11-28 |
 DURASAFE 73360322 1239457 Dead/Cancelled |
Industrial Canvas Products Co., Inc. 1982-04-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.