SOLOIST™
GUDID 00643169139510
CATH 441016U SOLOIST US W/O CE
MEDTRONIC, INC.
Cardiac mapping catheter, percutaneous, single-use| Primary Device ID | 00643169139510 |
| NIH Device Record Key | 98474ca9-5674-44ba-91f9-6b192c4c7b94 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | SOLOIST™ |
| Version Model Number | 441016U |
| Company DUNS | 006261481 |
| Company Name | MEDTRONIC, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
| Storage Environment Humidity | Between 0 Percent (%) Relative Humidity and 95 Percent (%) Relative Humidity |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00643169139510 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K953185
FDA Product Code
| DRF | CATHETER, ELECTRODE RECORDING, OR PROBE, ELECTRODE RECORDING |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-04-07 |
On-Brand Devices [SOLOIST™]
| 00643169139602 | CATH 44516U SOLOIST US W/O CE |
| 00643169139596 | CATH 44516JF SOLOIST US W/O CE |
| 00643169139558 | CATH 44216U SOLOIST US W/O CE |
| 00643169139541 | CATH 44216JF SOLOIST US W/O CE |
| 00643169139534 | CATH 44216J SOLOIST US W/O CE |
| 00643169139510 | CATH 441016U SOLOIST US W/O CE |
| 00643169139503 | CATH 441016JF SOLOIST US W/O CE |
| 00763000227609 | CATH 441016U SOLOIST W/O CE |
| 00763000227593 | CATH 44216J SOLOIST W/O CE |
| 00763000227586 | CATH 44216JF SOLOIST W/O CE |
| 00763000227579 | CATH 44216U SOLOIST W/O CE |
| 00763000227562 | CATH 44516J SOLOIST W/O CE |
| 00763000227555 | CATH 44516U SOLOIST W/O CE |
| 00763000227548 | CATH 448142J SOLOIST JOSEPH W/O CE |
Trademark Results [SOLOIST]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
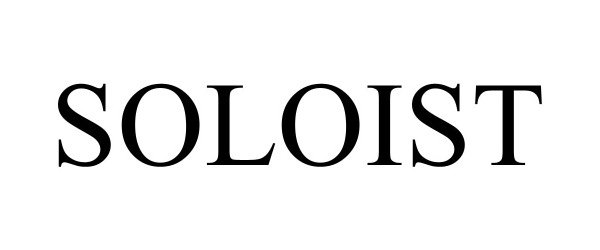 SOLOIST 98327917 not registered Live/Pending |
Starbuzz Tobacco, Inc. 2023-12-22 |
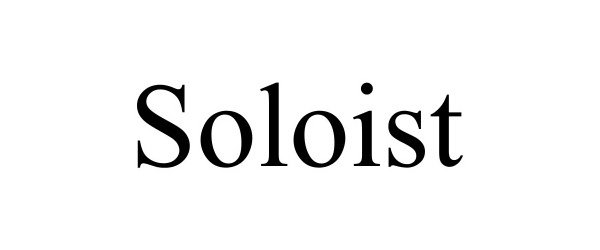 SOLOIST 98085490 not registered Live/Pending |
Busick, Parker E 2023-07-14 |
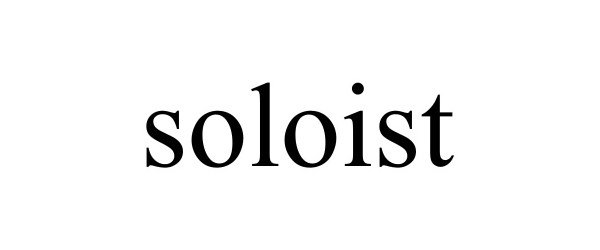 SOLOIST 97895203 not registered Live/Pending |
Liang Xuexing 2023-04-18 |
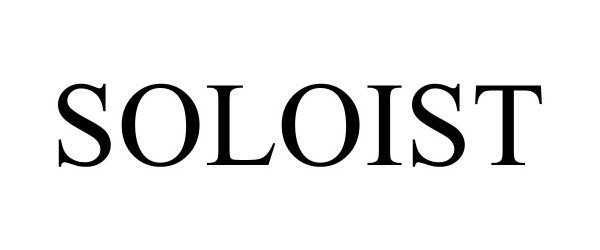 SOLOIST 97775423 not registered Live/Pending |
Conn-Selmer, Inc. 2023-01-31 |
 SOLOIST 97074203 not registered Live/Pending |
JACKSON/CHARVEL MANUFACTURING, INC. 2021-10-14 |
 SOLOIST 90618051 not registered Live/Pending |
Cervélo USA, Inc. 2021-04-01 |
 SOLOIST 87445455 not registered Live/Pending |
Adveris, LLC 2017-05-11 |
 SOLOIST 87443738 5449984 Live/Registered |
Adveris, LLC 2017-05-10 |
 SOLOIST 86268537 5161440 Live/Registered |
Precept Brands LLC 2014-05-01 |
 SOLOIST 78928276 3289115 Dead/Cancelled |
DIRECTED, LLC 2006-07-12 |
 SOLOIST 78642061 3313718 Live/Registered |
Boston Scientific Scimed, Inc. 2005-06-02 |
 SOLOIST 78417989 3007996 Live/Registered |
Aerotech, Inc. 2004-05-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.