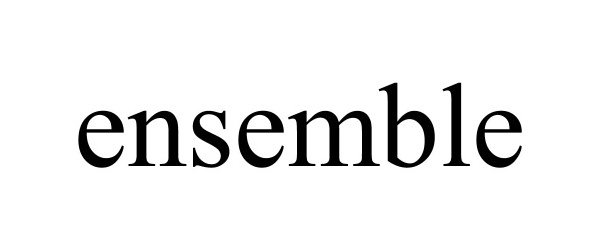| Primary Device ID | 00643169588448 |
| NIH Device Record Key | 5c403426-9973-408f-aa08-06009e59303b |
| Commercial Distribution Discontinuation | 2016-05-13 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | Ensemble™ |
| Version Model Number | NU1020 |
| Company DUNS | 006261481 |
| Company Name | MEDTRONIC, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |
| Special Storage Condition, Specify | Between 0 and 0 *Keep Away from Sunlight |