Black Maxx® Latex
GUDID 00649531130161
Latex Exam Glove, Black, Large
Dash Medical Gloves, Inc.
Hevea-latex examination/treatment glove, non-powdered, non-antimicrobial| Primary Device ID | 00649531130161 |
| NIH Device Record Key | 881ee899-750f-479f-81e7-a77be3ba54b7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Black Maxx® Latex |
| Version Model Number | BMX100L |
| Company DUNS | 194914768 |
| Company Name | Dash Medical Gloves, Inc. |
| Device Count | 100 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | true |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com | |
| Phone | 414-269-5298 |
| compliancedepartment@dashmedical.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00649531030065 [Unit of Use] |
| GS1 | 00649531030164 [Primary] |
| GS1 | 00649531130161 [Package] Contains: 00649531030164 Package: Case [10 Units] In Commercial Distribution |
FDA Product Code
| LYY | Latex Patient Examination Glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-09-30 |
| Device Publish Date | 2022-09-22 |
On-Brand Devices [Black Maxx® Latex]
| 00649531130178 | Latex Exam Glove, Black, X-Large |
| 00649531130161 | Latex Exam Glove, Black, Large |
| 00649531130147 | Latex Exam Glove, Black, Medium |
| 00649531130123 | Latex Exam Glove, Black, Small |
| 00649531130116 | Latex Exam Glove, Black, X-Small |
Trademark Results [Black Maxx]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
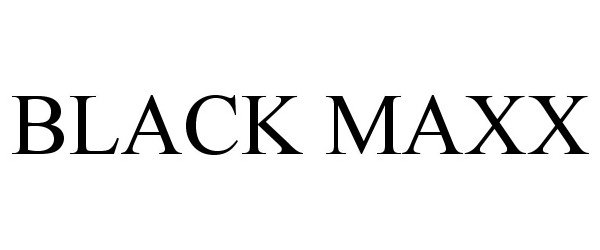 BLACK MAXX 85500799 4186580 Dead/Cancelled |
H & S Performance, LLC 2011-12-21 |
 BLACK MAXX 85377814 not registered Dead/Abandoned |
H&S Performance 2011-07-21 |
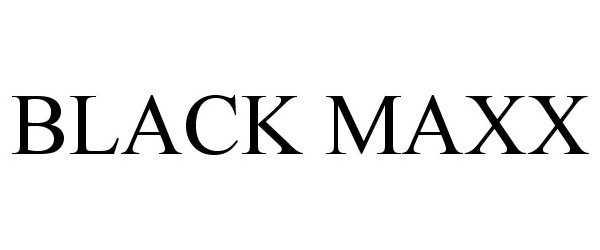 BLACK MAXX 85320614 4087006 Live/Registered |
DASH MEDICAL GLOVES, INC. 2011-05-13 |
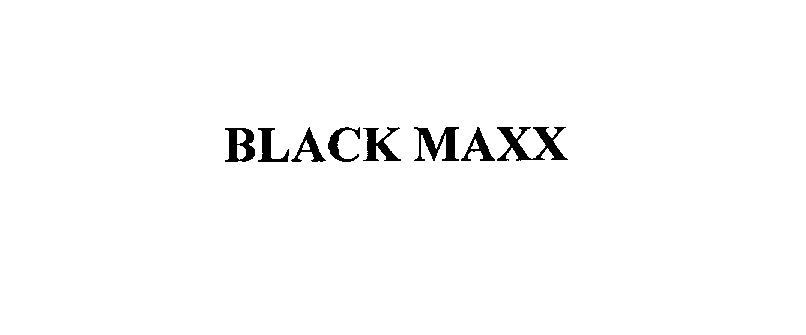 BLACK MAXX 76144478 2817805 Dead/Cancelled |
KEE Actions Sports II LLC 2000-10-10 |
 BLACK MAXX 75168146 2237966 Live/Registered |
CARTON CRAFT SUPPLY, INC. 1996-09-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.