Black Maxx® Thin Nitrile
GUDID 00649531141181
Nitrile Exam Glove, Black, XX-Large
Dash Medical Gloves, Inc.
Nitrile examination/treatment glove, non-powdered, non-antimicrobial| Primary Device ID | 00649531141181 |
| NIH Device Record Key | 8149dc0f-94f1-4d15-9499-9400f415658a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Black Maxx® Thin Nitrile |
| Version Model Number | BMNT200XXL |
| Company DUNS | 194914768 |
| Company Name | Dash Medical Gloves, Inc. |
| Device Count | 200 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com | |
| Phone | 4142695298 |
| compliancedepartment@dashmedical.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00649531041085 [Unit of Use] |
| GS1 | 00649531041184 [Primary] |
| GS1 | 00649531141181 [Package] Contains: 00649531041184 Package: Case [10 Units] In Commercial Distribution |
FDA Product Code
| LZA | Polymer Patient Examination Glove |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-07-24 |
| Device Publish Date | 2023-07-14 |
On-Brand Devices [Black Maxx® Thin Nitrile]
| 00649531141181 | Nitrile Exam Glove, Black, XX-Large |
| 00649531141174 | Nitrile Exam Glove, Black, X-Large |
| 00649531141167 | Nitrile Exam Glove, Black, Large |
| 00649531141143 | Nitrile Exam Glove, Black, Medium |
| 00649531141129 | Nitrile Exam Glove, Black, Small |
| 00649531141112 | Nitrile Exam Glove, Black, X-Small |
Trademark Results [Black Maxx]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
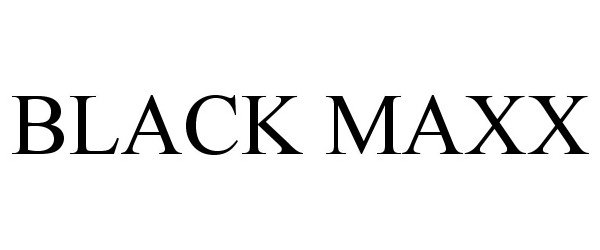 BLACK MAXX 85500799 4186580 Dead/Cancelled |
H & S Performance, LLC 2011-12-21 |
 BLACK MAXX 85377814 not registered Dead/Abandoned |
H&S Performance 2011-07-21 |
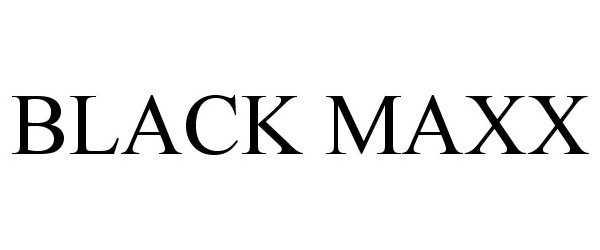 BLACK MAXX 85320614 4087006 Live/Registered |
DASH MEDICAL GLOVES, INC. 2011-05-13 |
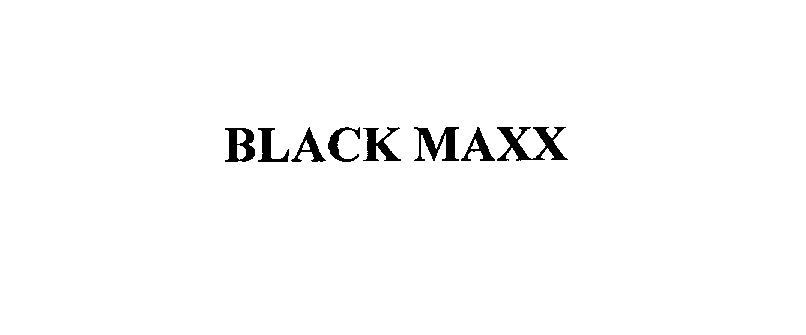 BLACK MAXX 76144478 2817805 Dead/Cancelled |
KEE Actions Sports II LLC 2000-10-10 |
 BLACK MAXX 75168146 2237966 Live/Registered |
CARTON CRAFT SUPPLY, INC. 1996-09-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.