RELY® H.Pylori 6300-020
GUDID 00657498000014
For the Qualitative Detection of IgG Antibodies to Helicobacter pylori (H. pylori) in Whole blood, Serum or Plasma.
STANBIO LABORATORY, L.P.
Helicobacter pylori immunoglobulin G (IgG) antibody IVD, kit, immunochromatographic test (ICT), rapid| Primary Device ID | 00657498000014 |
| NIH Device Record Key | 10c990ec-0fee-4043-9e75-f6c46e2177d7 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | RELY® H.Pylori |
| Version Model Number | 6300-020 |
| Catalog Number | 6300-020 |
| Company DUNS | 045361136 |
| Company Name | STANBIO LABORATORY, L.P. |
| Device Count | 20 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com | |
| Phone | 18005315535 |
| stanbiolab@ekfdiagnostics.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
| Storage Environment Temperature | Between 15 Degrees Celsius and 30 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00657498000014 [Primary] |
| GS1 | 00657498000021 [Unit of Use] |
FDA Product Code
| LYR | Helicobacter Pylori |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2016-09-23 |
Devices Manufactured by STANBIO LABORATORY, L.P.
| 00657498002094 - Uri-Trak 120 M Urine Analyzer | 2023-06-16 The Uri-Trak® 120 M Urine Analyzer is intended for use in conjunction with the Uri-Chek® 10SG Urinalysis Reagent Strips for th |
| 00657498002322 - HGB Controls - Low | 2021-04-08 A single level reference control intended for use on Hemo Control Hemoglobin Measuring system. |
| 00657498002339 - HGB Controls - Low | 2021-04-08 A single level reference control intended for use on Hemo Control Hemoglobin Measuring system. |
| 00657498002346 - HGB Controls - Normal | 2021-04-08 A single level reference control intended for use on Hemo Control Hemoglobin Measuring system. |
| 00657498002353 - HGB Controls - Normal | 2021-04-08 A single level reference control intended for use on Hemo Control Hemoglobin Measuring system. |
| 00657498002360 - HGB Controls - High | 2021-04-08 A single level reference control intended for use on Hemo Control Hemoglobin Measuring system. |
| 00657498002377 - HGB Controls - High | 2021-04-08 A single level reference control intended for use on Hemo Control Hemoglobin Measuring system. |
| 00657498001806 - Standard 1 | 2020-03-02 To provide calibration points for the Sodium, Potassium, and Chloride electrodes on the ISE module of Roche Cobas® Chemistry Sy |
Trademark Results [RELY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RELY 98838149 not registered Live/Pending |
Rely Innovations, Inc. 2024-11-05 |
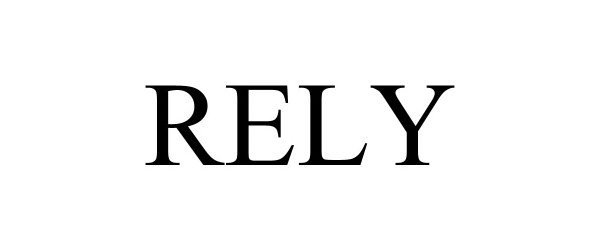 RELY 98377819 not registered Live/Pending |
Rely Innovations, Inc. 2024-01-26 |
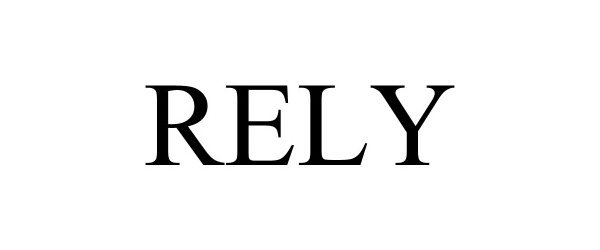 RELY 97917513 not registered Live/Pending |
Technip Energies France SAS 2023-05-02 |
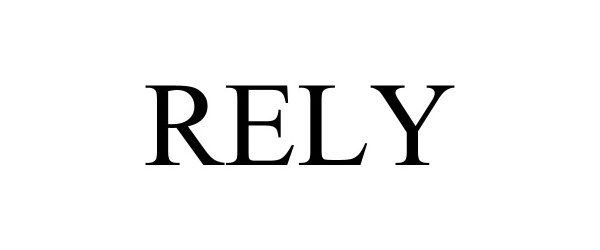 RELY 97707950 not registered Live/Pending |
J.R. Simplot Company 2022-12-07 |
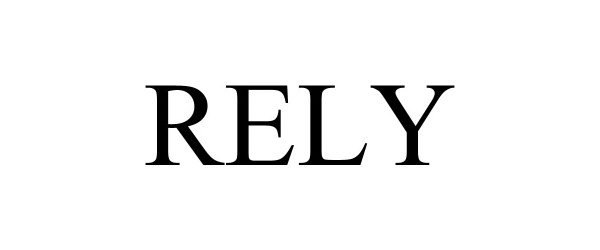 RELY 97702110 not registered Live/Pending |
Au Ratio Inc 2022-12-02 |
 RELY 97594744 not registered Live/Pending |
Rely Innovations, Inc. 2022-09-16 |
 RELY 97587387 not registered Live/Pending |
Kohler Co. 2022-09-12 |
 RELY 97526230 not registered Live/Pending |
UESCO, Inc. 2022-07-29 |
 RELY 88079241 not registered Live/Pending |
SpineFrontier, Inc. 2018-08-15 |
 RELY 88017034 not registered Dead/Abandoned |
SABIC Global Technologies B.V. 2018-06-27 |
 RELY 87930931 not registered Dead/Abandoned |
URO-1, INC. 2018-05-22 |
 RELY 87594024 not registered Dead/Abandoned |
RELY, LLC 2017-09-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.