INVISx®
GUDID 00681490881555
INSTRUMENT 60720 INVISX TRIMMING REU
MEDTRONIC PS MEDICAL, INC.
General internal orthopaedic fixation system implantation kit| Primary Device ID | 00681490881555 |
| NIH Device Record Key | f1546416-94c8-4d40-b000-f9816a5f6dbb |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | INVISx® |
| Version Model Number | 60720 |
| Company DUNS | 089055867 |
| Company Name | MEDTRONIC PS MEDICAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com | |
| Phone | +1(800)633-8766 |
| Corporate.UDI@medtronic.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00681490881555 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K010361
FDA Product Code
| GXN | PLATE, CRANIOPLASTY, PREFORMED, NON-ALTERABLE |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00681490881555]
Moist Heat or Steam Sterilization
[00681490881555]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2015-08-28 |
On-Brand Devices [INVISx®]
| 00643169103467 | INSTR 60710 INVISX TENSIONING REUSE |
| 00643169498242 | SET 60700 INVISX CRNL FXTION INSTR |
| 00643169498006 | INSTR 60710 INVISX TENSIONING REUSE |
| 00643169497771 | TRAY 60730 INVISX INSTRUMENT |
| 20643169466306 | LOCKS 60100 INVISX 2PK CE |
| 00681490881579 | TRAY 60730 INVISX INSTRUMENT |
| 00681490881555 | INSTRUMENT 60720 INVISX TRIMMING REU |
| 00681490881470 | SET 60700 INVISX CRNL FXTION INSTR |
Trademark Results [INVISx]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
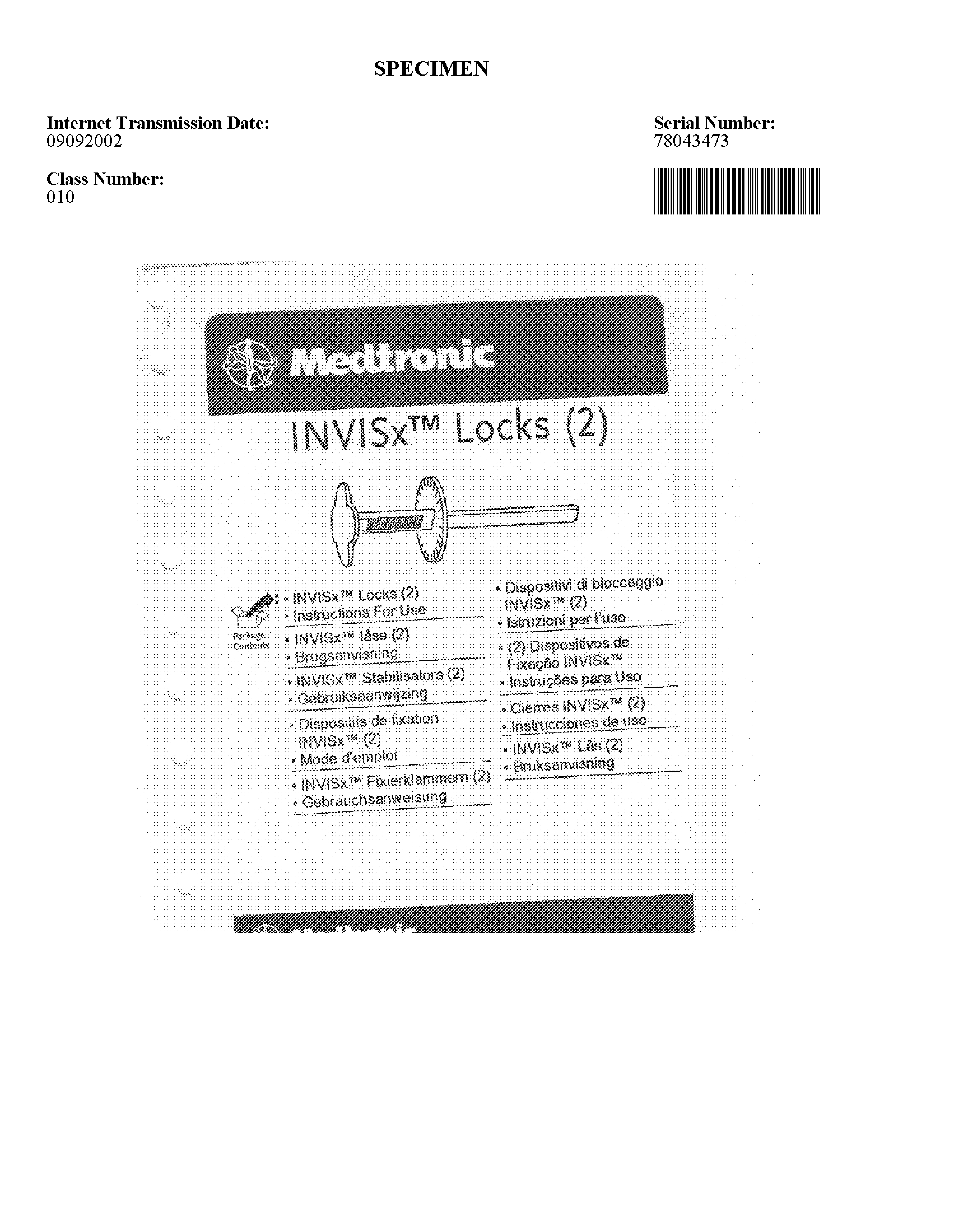 INVISX 78043473 2660838 Live/Registered |
Medtronic, Inc. 2001-01-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.