Regard
GUDID 00683534090343
A3PMSKUL4-REG AAMI Level 3, Procedure Mask, Earloop, Non Sterile, Universal Size, 50 Pcs/Dispenser
A PLUS INTERNATIONAL INC.
Surgical/medical face mask, non-antimicrobial, single-use| Primary Device ID | 00683534090343 |
| NIH Device Record Key | 4c88d636-2ca2-4759-aac7-f0868ecac66b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Regard |
| Version Model Number | A3PMSKUL4-REG |
| Company DUNS | 191705904 |
| Company Name | A PLUS INTERNATIONAL INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00683534090336 [Primary] |
| GS1 | 00683534090343 [Package] Contains: 00683534090336 Package: Dispenser [50 Units] In Commercial Distribution |
FDA Product Code
| FXX | Mask, Surgical |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-08-14 |
| Device Publish Date | 2025-08-06 |
Devices Manufactured by A PLUS INTERNATIONAL INC.
| 00683534090343 - Regard | 2025-08-14A3PMSKUL4-REG AAMI Level 3, Procedure Mask, Earloop, Non Sterile, Universal Size, 50 Pcs/Dispenser |
| 00683534090343 - Regard | 2025-08-14 A3PMSKUL4-REG AAMI Level 3, Procedure Mask, Earloop, Non Sterile, Universal Size, 50 Pcs/Dispenser |
| 80683534080616 - A Plus | 2024-07-25 SetUpCovr44x78 1760/GL;2.25mil,N-S,fan,N STL |
| 80683534040016 - A Plus | 2024-01-26 3M Hot Cold Pack - Nylon Cover, Bulk, 1000 PCS/CS |
| 80683534040023 - A Plus | 2024-01-26 3M Hot Cold Pack - Spunbond Polypropylene Cover (Tri-Fold), Bulk, 2000 PCS/CS |
| 80683534040030 - A Plus | 2024-01-26 3M Hot Cold Pack - #1572 Spunbond Polypropylene Cover, 100/CS |
| 00683534090282 - A Plus | 2023-08-14 Procedure Mask Earloop Green ASTM Level 1 |
| 00683534090305 - A Plus | 2023-08-14 Procedure Mask Earloop Green ASTM Level 3 |
| 00683534090329 - A Plus | 2023-08-14 Surgical Mask Tie-on Blue ASTM Level 3 |
Trademark Results [Regard]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
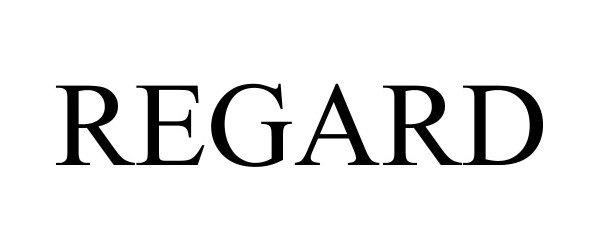 REGARD 97196978 not registered Live/Pending |
HealthTensor, Inc. 2021-12-30 |
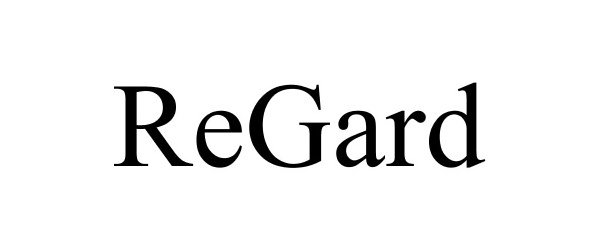 REGARD 86179619 4824780 Live/Registered |
MPC GROUP LLC 2014-01-30 |
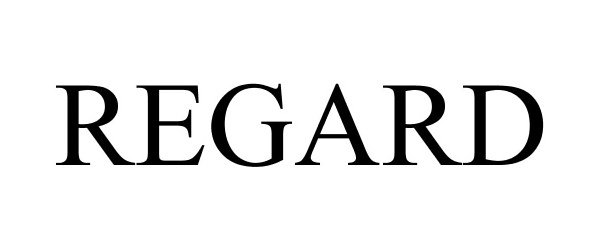 REGARD 86154589 4680759 Live/Registered |
LG Electronics Inc. 2013-12-30 |
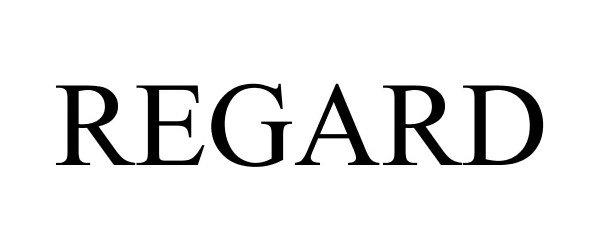 REGARD 85721657 4544205 Live/Registered |
Steelcase Inc. 2012-09-06 |
 REGARD 85342365 not registered Dead/Abandoned |
Clarke Mosquito Control Products, Inc. 2011-06-09 |
 REGARD 85236797 not registered Dead/Abandoned |
M. Z. Berger & Co., Inc. 2011-02-08 |
 REGARD 85025545 not registered Dead/Abandoned |
Syngenta Participations AG 2010-04-28 |
 REGARD 79033880 3340362 Dead/Cancelled |
Kreon, naamloze vennootschap 2006-12-07 |
 REGARD 78773486 not registered Dead/Abandoned |
Frederick Goldman, Inc. 2005-12-14 |
 REGARD 78064405 2675687 Dead/Cancelled |
Regard Ventures, LLC 2001-05-18 |
 REGARD 77864510 4289323 Live/Registered |
Resource Optimization & Innovation, LLC 2009-11-04 |
 REGARD 75742545 not registered Dead/Abandoned |
GARD CORPORATION 1999-10-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.