FLEX-TIP D98500
GUDID 00690103147421
FLEX-TIP TRANSLUMINAL A-PACING PROBE
Edwards Lifesciences LLC
Temporary cardiac pacing balloon catheter| Primary Device ID | 00690103147421 |
| NIH Device Record Key | 47b8824f-ed9a-4242-b855-f09845c80985 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | FLEX-TIP |
| Version Model Number | D98500 |
| Catalog Number | D98500 |
| Company DUNS | 134139174 |
| Company Name | Edwards Lifesciences LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Unsafe |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)822-9637 |
| tech_support@edwards.com | |
| Phone | +1(800)822-9637 |
| tech_support@edwards.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *STORE IN A COOL, DRY PLACE. |
| Special Storage Condition, Specify | Between 0 and 0 *STORE IN A COOL, DRY PLACE. |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00690103147421 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K152633
FDA Product Code
| LDF | ELECTRODE, PACEMAKER, TEMPORARY |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-03 |
Devices Manufactured by Edwards Lifesciences LLC
| 00690103221312 - Edwards SAPIEN 3 transcatheter pulmonic valve delivery system | 2026-02-25 Pulmonic Delivery System - 20 mm (Gen 2) (US) |
| 00690103221329 - Edwards SAPIEN 3 transcatheter pulmonic valve delivery system | 2026-02-25 Pulmonic Delivery System - 23 mm (Gen 2) (US) |
| 00690103221336 - Edwards SAPIEN 3 transcatheter pulmonic valve delivery system | 2026-02-25 Pulmonic Delivery System - 26 mm (Gen 2) (US) |
| 00690103221343 - Edwards SAPIEN 3 transcatheter pulmonic valve delivery system | 2026-02-25 Pulmonic Delivery System - 29 mm (Gen 2) (US) |
| 00690103019353 - AORTIC TRUE-SIZE OBTURATOR 19MM | 2026-02-23 AORTIC TRUE-SIZE OBTURATOR F/USE W/1108 HDLE |
| 00690103019360 - AORTIC TRUE-SIZE OBTURATOR 21MM | 2026-02-23 AORTIC TRUE-SIZE OBTURATOR F/USE W/1108 HDLE |
| 00690103019377 - AORTIC TRUE-SIZE OBTURATOR 23MM | 2026-02-23 AORTIC TRUE-SIZE OBTURATOR F/USE W/1108 HDLE |
| 00690103019384 - AORTIC TRUE-SIZE OBTURATOR 25MM | 2026-02-23 AORTIC TRUE-SIZE OBTURATOR F/USE W/1108 HDLE |
Trademark Results [FLEX-TIP]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
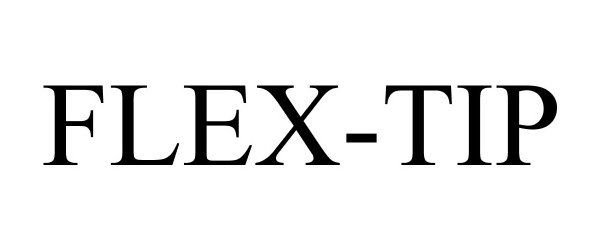 FLEX-TIP 78749045 not registered Dead/Abandoned |
Parker Medical, Inc. 2005-11-08 |
 FLEX-TIP 73706340 not registered Dead/Abandoned |
TARGET THERAPEUTICS, INC. 1988-01-19 |
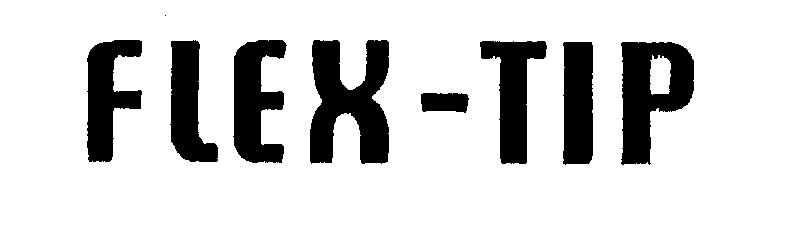 FLEX-TIP 71656792 0603486 Live/Registered |
HECKETHORN MANUFACTURING & SUPPLY CO. 1953-11-23 |
 FLEX-TIP 71621498 0590311 Dead/Expired |
BAKER BRUSH CO., INC. 1954-03-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.